This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Probe expands understanding of oral cavity homeostasis
Your mouth is a crucial interface between the outside world and the inside of your body. Everything you breathe, chew, or drink interacts with your oral cavity—the proteins and the microbes, including microbes that can harm us. When things go awry, the result can range from the mild, like bad breath, to the serious, like tooth and gum decay, to more dire effects in the gut and other parts of the body.
Even though the oral microbiome plays a critical role as a front-line defense for human health and disease, we still know very little about the intricacies of host-microbe interactions in the complex physiological environment of the mouth; a better understanding of those interactions is key to developing treatments for human disease.
In a recent study published in PNAS, a team of scientists from MIT and elsewhere revealed that one of the most abundant proteins found in our saliva binds to the surface of select microbes found in the mouth. The findings shed light on how salivary proteins and mucus play a role in maintaining the oral cavity microbiome.
The collaboration involved members of the labs of Barbara Imperiali in the MIT Department of Biology and Laura Kiessling in the MIT Department of Chemistry, as well as the groups of Stefan Ruhl at the University at Buffalo School of Dental Medicine and Catherine Grimes at the University of Delaware.
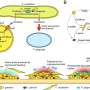
The work is focused on an abundant oral cavity protein called zymogen granule protein 16 homolog B (ZG16B). Finding ZG16B's interaction partners and gaining insight into its function were the overarching goals of the project. To accomplish this, Soumi Ghosh, a postdoc in the Imperiali lab, and colleagues engineered ZG16B to add reporter tags such as fluorophores. They called these modified proteins "microbial glycan analysis probes (mGAPs)" because they allowed them to identify ZG16B binding partners using complementary methods. They applied the probes to samples of healthy oral microbiomes to identify target microbes and binding partners.
The results excited them.
"ZG16B didn't just bind to random bacteria. It was very focused on certain species, including a commensal bacteria called Streptococcus vestibularis," says Ghosh, who is first author on the paper.
Commensal bacteria are found in a normal healthy microbiome and do not cause disease.
Using the mGAPs, the team showed that ZG16B binds to cell wall polysaccharides of the bacteria, which indicates that ZG16B is a lectin, a carbohydrate-binding protein. In general, lectins are responsible for cell-cell interactions, signaling pathways, and some innate immune responses against pathogens. "This is the first time that it has been proven experimentally that ZG16B acts as a lectin because it binds to the carbohydrates on the cell surface or cell wall of the bacteria," Ghosh highlights.
ZG16B was also shown to recruit Mucin 7 (MUC7), a salivary glycoprotein in the oral cavity, and together the results suggest that ZG16B may help maintain a healthy balance in the oral microbiome by forming a complex with MUC7 and certain bacteria. The results indicate that ZG16B regulates the bacteria's abundance by preventing overgrowth through agglutination when the bacteria exceed a certain level of growth.
"ZG16B, therefore, seems to function as a missing link in the system; it binds to different types of glycans—the microbial glycans and the mucin glycans—and ultimately, maintains a healthy balance in our oral cavity," Ghosh says.
Further work with this probe and samples of oral microbiome from healthy and diseased subjects could also reveal the lectin's importance for oral health and disease.
Current attention is focused on developing and applying additional mGAPs based on other human lectins, such as those found in serum, liver, and intestine to reveal their binding specificities and their roles in host-microbe interactions.
"The research carried out in this collaboration exemplifies the kind of synergy that made me excited to move to MIT five years ago," says Kiessling. "I've been able to work with outstanding scientists who share my interest in the chemistry and the biology of carbohydrates."
Kiessling and Imperiali, both senior authors of the paper, came up with the term for the probes they're creating: "mGAPS to fill in the gaps" in our understanding of the role of lectins in the human microbiome, according to Ghosh.
"If we want to develop therapeutics against bacterial infection, we need a better understanding of host-microbe interactions," Ghosh says. "The significance of our study is to prove that we can make very good probes for microbial glycans, find out their importance in the front-line defense of the immune system, and, ultimately, come up with a therapeutic approach to disease."
More information: Soumi Ghosh et al, Human oral lectin ZG16B acts as a cell wall polysaccharide probe to decode host–microbe interactions with oral commensals, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2216304120
Journal information: Proceedings of the National Academy of Sciences
Provided by Massachusetts Institute of Technology
This story is republished courtesy of MIT News (web.mit.edu/newsoffice/), a popular site that covers news about MIT research, innovation and teaching.