This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
What drives ions through polymer membranes?

Ion exchange membranes are needed in (photo)electrolyzers, fuel cells and batteries to separate ions and enable the desired processes. Polymeric membranes such as synthetically produced compounds like NAFION are particularly efficient, but they cannot be degraded. A ban on the use of these "eternal chemicals" is currently under discussion in the European Union, and the development of suitable alternatives will be a major challenge. So, it is crucial to understand why NAFION and other established polymeric membranes work so well.
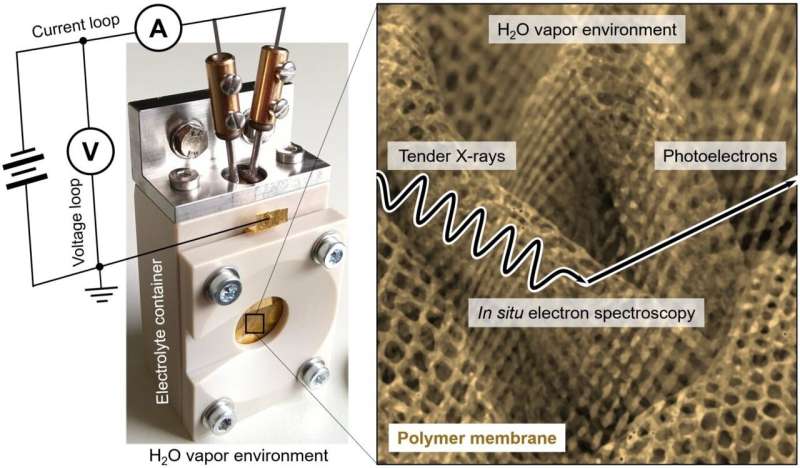
A team led by Dr. Marco Favaro of the HZB Institute for Solar Fuels has now investigated this using a special type of electrolysis cell. Here, the membrane sits on the outer wall and is in contact with both the liquid electrolyte and a gaseous external environment. It can act either as an anode or a cathode, depending on the polarity of the applied potential.
This hybrid liquid-gas electrolyzer is considered particularly favorable for the electrochemical conversion of CO2 thanks to the higher CO2 concentrations that can be achieved in the gas phase, thereby overcoming the poor solubility of CO2 in aqueous solutions.
For the study, Favaro and his team used commercially available ion-exchange membranes in contact with a model electrolyte like sodium chloride (NaCl) in water. Water vapor was fed to the gas phase, with the partial pressure of water close to its vapor pressure at room temperature. To analyze the migration of sodium and chloride ions through the membrane, they used in situ ambient pressure hard X-ray photoelectron spectroscopy (AP-HAXPES) at the SpAnTeX end-station at the KMC-1 beamline of BESSY II.
"Indeed, we were expecting that the ion dynamics was determined, under applied potentials, by the electric fields generated between the anode and cathode of the electrolyzer, and that electromigration was therefore the main driver," says Marco Favaro.
However, analysis of the data showed otherwise: electromigration hardly plays a role; the ions simply diffuse across the membrane. The data could be perfectly simulated numerically with a diffusion model. "Our conclusion is that ions move through the polymer membranes in these types of electrolyzers due to hopping mediated by the ionized functional groups present in the membranes. In addition, since water diffuses as well through the polymer, the ions are 'dragged' as well," explains Favaro.
These results are exciting for a number of reasons: These types of electrolyzers are a way to convert CO2 into valuable chemicals that can otherwise only be obtained from fossil fuels. Understanding how these devices work helps on the way to decarbonize the economy. On the other hand, the ion-exchange membranes that are a key component of these cells are themselves problematic: the European Union may soon ban the use of persistent chemicals.
Understanding the relevant drivers of such transport processes will help to develop new membrane materials that are both efficient, durable, and environmentally friendly. Favaro now intends to take this project forward at HIPOLE, the new Helmholtz Institute in Jena, which will focus on polymer materials for new energy technologies.
The paper is published in the Journal of Materials Chemistry A.
More information: Maryline Ralaiarisoa et al, In situ investigation of ion exchange membranes reveals that ion transfer in hybrid liquid/gas electrolyzers is mediated by diffusion, not electromigration, Journal of Materials Chemistry A (2023). DOI: 10.1039/D3TA02050A
Journal information: Journal of Materials Chemistry A
Provided by Helmholtz Association of German Research Centres