This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A new Achilles heel of the bacterial cell wall
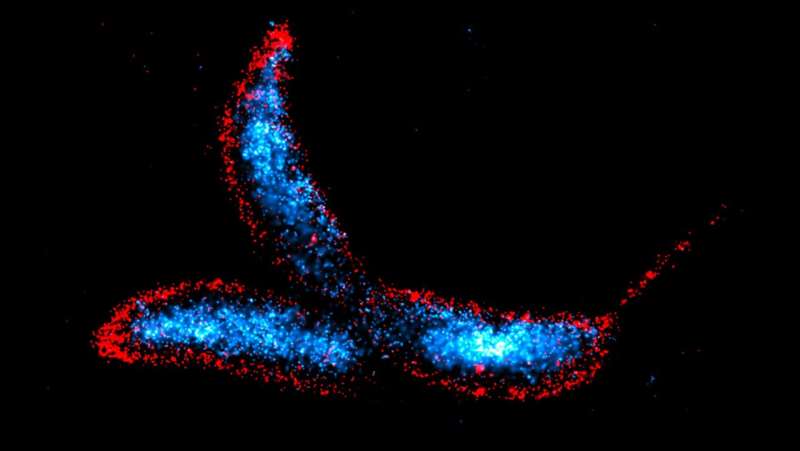
The bacterial cell wall must be constantly remodeled in order to grow and divide. This involves the close coordination of lytic enzymes and peptidoglycan synthesis. In their study published in Nature Communications, researchers led by Martin Thanbichler have now found that a central regulator can control completely different classes of autolysins. Since many antibiotics target the bacterial cell wall, these findings may contribute to the development of new therapeutic strategies against bacterial infections.
During evolution, cells have developed a wide range of strategies to strengthen their envelope against internal osmotic pressure, thus allowing them to grow in a variety of different environments.
Most bacterial species synthesize a semi-rigid cell wall surrounding the cytoplasmic membrane, whose main component, peptidoglycan, forms a dense meshwork that encases the cell. In addition to its protective role, the cell wall also serves as a means to generate specific cell shapes, such as spheres, rods, or spirals, thus facilitating motility, surface colonization, and pathogenicity.
The presence of a cell wall presents its own challenges: cells must constantly remodel it in order to grow and divide. To do this, they must very carefully make tears in the wall to allow it to expand and change, while quickly mending the gaps with new material to prevent it from collapsing.
This cell wall remodeling process involves the cleavage of bonds by lytic enzymes, also known as autolysins, and the subsequent insertion of new cell wall material by peptidoglycan synthases. The activities of these two antagonistic groups of proteins must be closely coordinated to prevent weak spots in the peptidoglycan layer that lead to cell lysis and death.
The research team led by Martin Thanbichler, Max Planck Fellow at the Max Planck Institute for Terrestrial Microbiology and Professor of Microbiology at the University of Marburg, has set out to unravel the composition and function of the autolytic machinery. Their studies focus on the crescent-shaped bacterium Caulobacter crescentus, which is found in freshwater environments and widely used as a model organism to study fundamental cellular processes in bacteria.
According to Thanbichler, studying the function of autolysins has been a challenging task. "While we know a lot about the synthetic machinery, the autolysins proved to be a tough nut to crack." Maria Billini, a postdoctoral researcher in Thanbichler's team, adds, "Bacteria usually harbor many types of autolysins from different enzyme families with different targets. This means that these proteins are highly redundant, and the deletion of individual autolysin genes often has little effect on cell morphology and growth."
Versatile regulator
Analysis of potential autolysin regulators by co-immunoprecipitation screening and in vitro protein-protein interaction assays has revealed that a factor called DipM plays a pivotal role in bacterial cell wall remodeling. This key regulator, a soluble periplasmic protein, surprisingly interacts with several classes of autolysins as well as a cell division factor, showing a promiscuity that was previously unknown for this type of regulator.
DipM was able to stimulate the activity of two peptidoglycan-cleaving enzymes with completely different activities and folding, making it the first identified regulator that can control two classes of autolysins. Notably, the results also indicate that DipM uses a single interface to interact with its various targets.
"Disruption of DipM leads to the loss of regulation at various points of the cell wall remodeling and division process and ultimately kills the cell," says doctoral student Adrian Izquierdo Martinez, first author of the study. "Its proper function as a coordinator of autolysin activity is thus critical for proper cell shape maintenance and cell division in C. crescentus."
The comprehensive characterization of DipM revealed a novel interaction network, including a self-reinforcing loop that connects lytic transglycosylases and possibly other autolysins to the core of the cell division apparatus of C. crescentus, and very likely also other bacteria. Thus, DipM coordinates a complex autolysin network whose topology greatly differs from that of previously studied autolysin systems.
Martin Thanbichler points out, "The study of such multi-enzyme regulators, whose malfunction affects several cell wall-related processes at the same time, not only helps us to understand how the cell wall responds to changes in the cell or the environment. It can also contribute to the development of new therapeutic strategies that combat bacteria by disrupting several autolytic pathways simultaneously. "
More information: Adrian Izquierdo-Martinez et al, DipM controls multiple autolysins and mediates a regulatory feedback loop promoting cell constriction in Caulobacter crescentus, Nature Communications (2023). DOI: 10.1038/s41467-023-39783-w
Journal information: Nature Communications
Provided by Max Planck Society