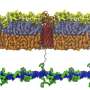
Potential weakness in the protective layers surrounding Gram-negative bacteria
A new study published in Nature today has identified a potential Achilles heel in the protective layers surrounding Gram-negative bacteria that could aid in the development of next-generation antibiotics. The study, carried out jointly by Professor Waldemar Vollmer and Dr. Federico Corona at Newcastle University, alongside Professor Colin Kleanthous and Dr. Gideon Mamou in the Department of Biochemistry at the University of Oxford, shows that Gram-negative bacteria depend on the cell wall to synchronize building of the outer membrane.
The World Health Organization (WHO) has declared Antimicrobial Resistance (AMR) one of the top 10 global public health threats. Some bacteria have already become resistant to all known antibiotics. Particularly problematic are multidrug resistant Gram-negative bacteria such as Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae that cause pneumonias and sepsis. Their outer membrane resides beyond the cell wall and excludes many classes of antibiotics that would otherwise target it.
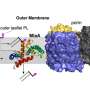
The research reveals that the cell wall, which is composed of a tough material known as peptidoglycan, exerts surprising control over where new proteins are introduced into the outer membrane by an essential biogenesis protein known as BamA. This helps bacteria coordinate these layers, which is crucial for the way they grow.
Professor Vollmer from the Center for Bacterial Cell Biology at Newcastle University Biosciences Institute explains that "bacteria are tiny yet have a very high internal pressure, like a car tire. They have a strong cell wall to withstand this pressure and prevent them bursting while still allowing them to grow and divide. What we have revealed for the first time is as they grow, how the process of wall expansion is linked to that of the outer membrane beyond it."
The research revealed that the age of the mesh-like peptidoglycan—composed of amino acids and sugars—is the key factor. "Old" peptidoglycan that occurs primarily at the poles (ends) of cells completely shuts down new outer membrane protein biogenesis. By contrast, "new" peptidoglycan that occurs primarily at sites where cells are about to divide, allows the greatest amount of outer membrane growth. This simple yet elegant mechanism ensures bacteria maintain a tight control over both cell envelope layers.
Professor Kleanthous from Oxford added that they "never suspected that Gram-negative bacteria were so reliant on the cell wall to coordinate growth of the outer membrane. Disrupting this cross-talk would literally 'open up' Gram-negative bacteria, making them vulnerable to antibiotics that the outer membrane otherwise excludes."
More information: Waldemar Vollmer, Peptidoglycan maturation controls outer membrane protein assembly, Nature (2022). DOI: 10.1038/s41586-022-04834-7. www.nature.com/articles/s41586-022-04834-7
Journal information: Nature
Provided by Newcastle University