This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Prepared for war: How cells survive viral invasion
"Let him who desires peace, prepare for war," wrote the Roman author Vegetius in the 4th century CE. Our bodies, it seems, live by this dictum: Even in times of peace, some cells express high levels of defensive, antiviral proteins. A new Weizmann Institute of Science study published in Nature Microbiology reveals that the higher the routine levels of these proteins in a cell, the greater the chances of preventing a viral takeover.
When a virus enters a cell, it can gain control of the protein production mechanisms, forcing them to make multiple copies of itself. But infection might not lead to all-out war. While an active infection entails the virus killing the cell as it spreads its copies to other cells, at times a virus fails to take over the production mechanisms of the host cell, remaining latent within it, sometimes for decades. Herpes viruses, for example, are notorious for their ability to hide inside the body in a dormant state. What determines whether an active infection occurs, or the virus remains latent?
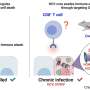
A team of researchers led by Dr. Michal Schwartz of Prof. Noam Stern-Ginossar's lab in Weizmann's Molecular Genetics Department has addressed this question by focusing on human cytomegalovirus, a member of the herpes family that infects most of the population.
Like other herpes viruses, cytomegalovirus awaits silently in the bodies of carriers, although it might cause an onset of symptomatic illness anytime later in life. The virus often raises its ugly head when a patient is immunosuppressed, following an organ transplant or chemotherapy. Pregnant women contracting the virus can pass it to the fetus, which sometimes suffers from serious illness as a result.
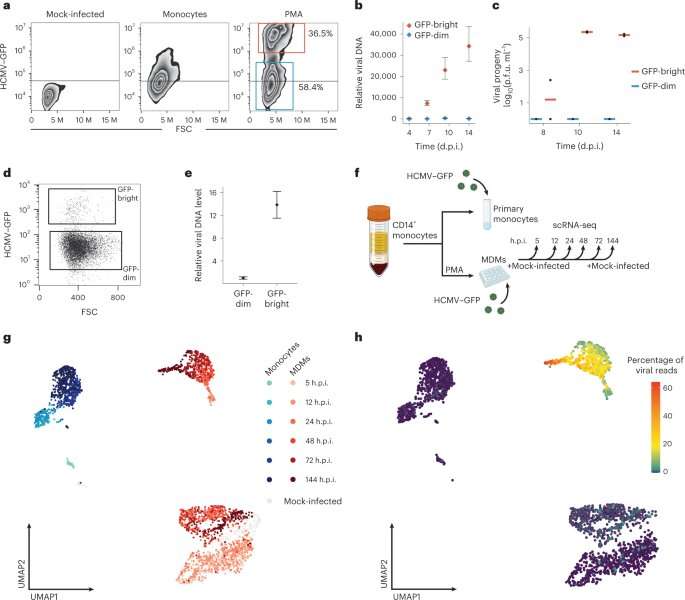
The scientists monitored the process of infection in two groups of immune cells—macrophages and their precursor cells—for 144 hours. At several points in time after infection, they sequenced RNA molecules from individual cells. Since RNA molecules carry the recipes for protein production, sequencing them revealed which proteins are produced, and in what quantities, at each stage of infection.
"As expected, shortly after infection the cells still only produced their own proteins," says Schwartz. "However, a few hours later, the cells split into two groups. As some kept making their own proteins, others started assembling viral proteins, a step that initiates the multiplication of the viral genome and its spread throughout the body. We found this step to be irreversible: From the moment cells expressed just two initial viral proteins, we couldn't stop the viral takeover."
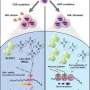
Searching for an explanation as to why the virus took over some cells but not others, the team examined the differences in proteins produced by each group of cells before and during infection. They discovered that the virus failed to take over cells that had already produced more antiviral defenses prior to infection. While the virus stayed as a latent guest within these cells, those with a lower routine production of defense proteins were open to a viral takeover.
This local protein "shield," produced not only by the attacked cell but throughout its environment, had long been recognized as the first line of defense against an ongoing viral infection. It was known to be generated in response to viral invasion, which prompts cells to secrete protein alerts called interferons. These warning signs, in turn, elicit antiviral protein production in nearby cells—a sort of code red to get ready for battle. Following the secretion of interferons, hundreds of genes for defense proteins are activated. The team's finding showed that these defense proteins play a significant role even before the infection occurs and the warning signs are flagged.
The researchers believe that high levels of routine defense protein production might serve to immunize cells against a potential active infection. This idea suggests a possible solution to a previously unsolved mystery—why do cytomegaloviruses tend to accumulate in a latent state within bone marrow stem cells? Previous research by other scientists had found that stem cells routinely produce relatively high levels of defense proteins, compared to such mature immune cells as macrophages. These proteins' newly discovered immunizing effect against active infection could explain why the viruses remain latent within the stem cells.
The scientists also discovered that mature macrophages, which had been thought to only play host to active infection, can harbor latent viral infection as well. This means that contrary to the prevailing view, cells don't fall into one of two categories—harboring either active or latent infection—but can be host to either, depending on their levels of defense protein production. In other words, various cell types in the body, formerly thought to be subject to active infection alone, might in fact form unknown reservoirs of latent, potentially harmful viruses. Discovering such reservoirs can lead to preventive treatments.
"Latent viruses pose an existential threat to immunosuppressed patients and organ transplant recipients," says Stern-Ginossar. "A basic understanding of the mechanisms that determine whether an onset of illness occurs or the virus remains latent is crucial to developing effective treatments. If we find ways to activate or deactivate latent viruses on demand, it will allow us to better prepare patients prior to transplant or treatment. I am hopeful that the understanding of cellular self-defense mechanisms will find its way to effective solutions in the clinical field."
The following researchers also participated in the study: Dr. Miri Shnayder, Aharon Nachshon, Tamar Arazi, Yaarit Kitsberg, Roi Levi Samia and Michael Lavi of Weizmann's Molecular Genetics Department; and Dr. Rottem Kuint and Dr. Reuven Tsabari of Hadassah University Medical Center.
More information: Michal Schwartz et al, Molecular characterization of human cytomegalovirus infection with single-cell transcriptomics, Nature Microbiology (2023). DOI: 10.1038/s41564-023-01325-x
Journal information: Nature Microbiology
Provided by Weizmann Institute of Science