This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Unlocking the potential of enantioselective catalysis: Advancements in pyrrolidinyl gold(I) complexes
Remarkable strides in the field of enantioselective catalysis have been made by investigating the unique properties of pyrrolidinyl gold(I) complexes.
Researchers from the Prof. Echavarren group at the Institute of Chemical Research of Catalonia (ICIQ-CERCA) in collaboration with Dr. Maria Besora from the URV in Tarragona have published in JACS Au their pioneering study, employing a combination of Density Functional Theory (DFT) calculations and the newly developed NEST App.
The findings pave the way for enhanced understanding of electronic and steric effects and expedite the design of novel chiral ligands for enantioselective reactions.
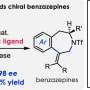
Building upon the success of their previous work with chiral gold(I) catalysts, the research team focused on exploring the impact of structural modifications on electronic and steric effects of the ligands. To this end, they synthesized more than 20 new gold(I) complexes and evaluated their performance in two key reactions: the enantioselective formal [4+2] cycloaddition of arylalkynes with alkenes and the atroposelective synthesis of 2-arylindoles through the cyclization of sulfonamidyl diarylacetylenes.
Remarkably, the novel catalysts exhibited excellent enantioselectivities, highlighting the potential of the pyrrolidinyl gold(I) complex scaffold.
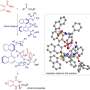
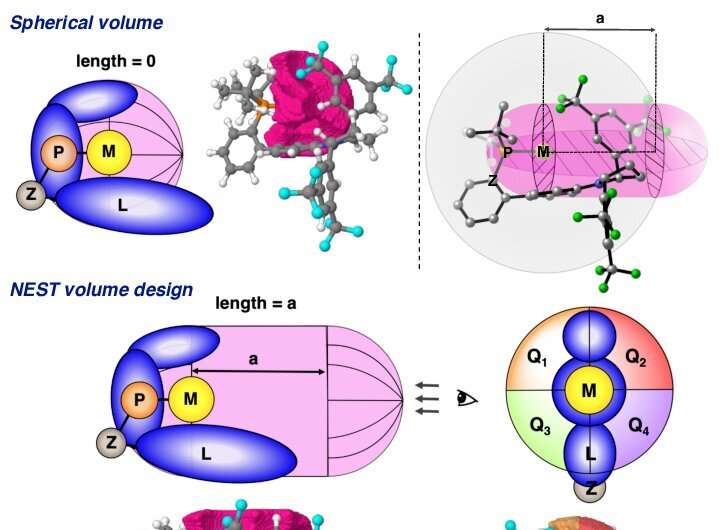
The computational work carried out by Dr. Imma Escofet, in the group of Prof. Echavarren, in collaboration with Dr. Besora was crucial for developing a cutting-edge open-source tool called NEST App. This innovative software computes the volume occupied by the atoms of a molecule within a defined capsule-shaped region, enabling the detailed investigation of steric effects within the binding pockets of gold(I) catalysts.
The data generated by the NEST App provided valuable insights and facilitated the prediction of experimental enantioselectivities, exhibiting reasonable agreement with the actual experimental results. This new tool accelerates the process of designing new chiral ligands, significantly reducing the time required for optimization.
The study conducted by the team in Tarragona not only advances our fundamental understanding of enantioselective catalysis with pyrrolidinyl gold(I) complexes but also establishes a powerful platform for the future development of highly efficient and selective catalysts. These findings hold tremendous potential for revolutionizing the field and driving transformative advancements in the synthesis of complex chiral molecules.
More information: Giuseppe Zuccarello et al, Enantioselective Catalysis with Pyrrolidinyl Gold(I) Complexes: DFT and NEST Analysis of the Chiral Binding Pocket, JACS Au (2023). DOI: 10.1021/jacsau.3c00159
Provided by Institute of Chemical Research of Catalonia