June 19, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Metallic bond between two beryllium atoms in ligand framework made for the first time
A quartet of chemists at the University of Oxford has, for the first time, found a way to get two beryllium atoms to bond with one another in a complex compound. In their paper published in the journal Science, Josef Boronski, Agamemnon Crumpton, Lewis Wales and Simon Aldridge, describe their process and how they managed to do it in a safe way—and at room temperature. Jason Dutton with La Trobe University, has published a Perspective piece in the same journal issue, outlining the work done by the team in England.
Beryllium is a strong but lightweight, alkaline earth metal. It is also brittle.
Beryllium only ever occurs naturally when mixed with other elements, forming minerals. It is often found in gemstones such as emeralds. And it is used in a variety of applications, from telecommunications equipment to computers and cell phones. It is also mixed with other metals to create alloys used in applications such as gyroscopes and electrical contacts.
For many years, scientists have thought that the element could be even more useful if a way could be found to force beryllium atoms to bond with one another. But until now, it was not possible.
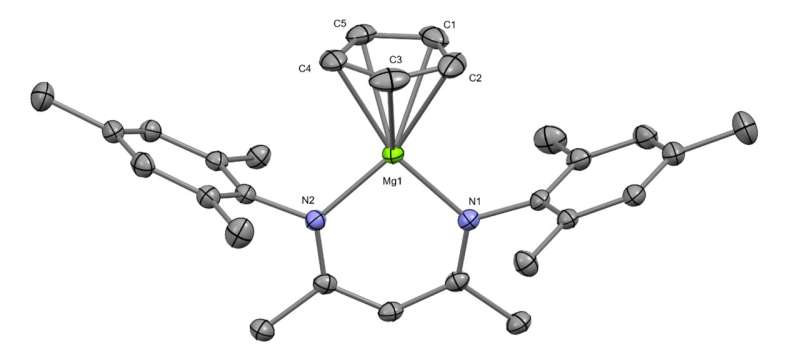
Getting two beryllium atoms to bond involved developing a multi-stage process. The first step involved building two molecules with each made up of a single beryllium atom and several carbon and hydrogen atoms. The team then mixed the two molecules together with a chemical made by bonding dual magnesium atoms together. Doing so caused a reaction to occur that involved the donation of electrons, resulting in the creation of a crystal compound called diberyllocene. A closeup look at the crystal structure showed that it contained the original beryllium atoms bonded with one another at its center. The team also showed that the compound reacted with iodides, forming bonds with zinc and aluminum.
The research team notes that one of the things that prevented others from bonding beryllium atoms was the toxicity involved. But they found that by first establishing a group of safety protocols, they could conduct their procedure in a safe manner. They note that part of that protocol involved making the new compound quickly and very cleanly. They also found that math models used to make predictions decades ago—showing the resulting compound to be stable—proved to be correct.
Dutton notes that showing that beryllium atoms can be bonded should lead to a better understanding of such bonding in general, and in particular, the nature of beryllium atoms when they bond.
More information: Josef T. Boronski et al, Diberyllocene, a stable compound of Be(I) with a Be–Be bond, Science (2023). DOI: 10.1126/science.adh4419
Jason L. Dutton, A big breakthrough for beryllium, Science (2023). DOI: 10.1126/science.adi5762
Journal information: Science
© 2023 Science X Network