High pressure creates new neighbours for beryllium
The rare element beryllium is mainly known for being a component of emeralds, aquamarines, and other precious stones. However, in Nature Communications, an international team of scientists from the University of Bayreuth now reports on a very unusual discovery: Under a pressure 880,000 times higher than the pressure of the Earth's atmosphere, beryllium atoms in a phosphate crystal surround themselves with six neighbouring atoms instead of the usual four. Actually, this crystal structure was theoretically predicted five decades ago, but it was only during high-pressure experiments at the Deutsches Elektronensynchrotron (DESY) in Hamburg that it has now been observed for the first time.
Prof. Dr. Leonid Dubrovinsky and Dr. Maxim Bykov from the Bavarian Geo Institute were involved in the research work on the part of the University of Bayreuth, as were Georgios Aprilis and Dr. Anna Pakhomova from the Working Group for Material Physics and Technology under Extreme Conditions in the Laboratory for Crystallography.
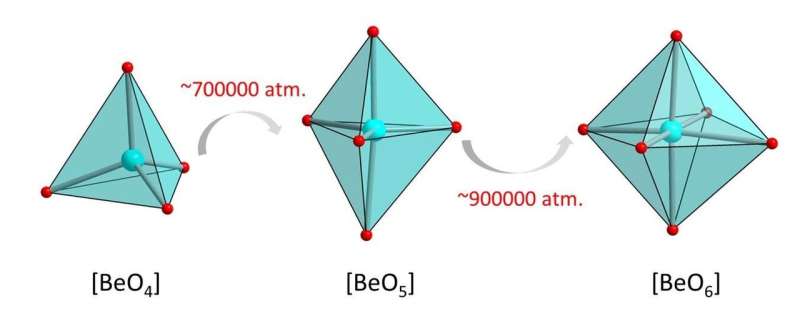
Originally, science considered it impossible for beryllium atoms in crystals to have more than four neighbouring atoms. This seemed to be incompatible with the crystal-chemical laws for a long time. "But about 50 years ago, theorists suggested that higher co-ordinations might actually be possible, even though these have stubbornly eluded experimental confirmation in inorganic compounds ever since," reports Dr. Anna Pakhomova, a beamline scientist at DESY and post-doctoral researcher preparing a habilitation thesis at Bayreuth University.
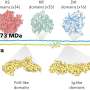
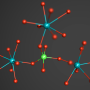
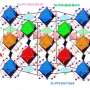
High-pressure experiments at DESY's X-ray light source PETRA III have made empirical proof possible for the first time. The researchers examined samples of the phosphate crystal hurlbutite, a rare mineral consisting of calcium, beryllium, phosphorus and oxygen (CaBe2P2O8), which occurs naturally on the earth's surface. Under normal environmental conditions, each beryllium atom only has four oxygen atoms as neighbours. At 700,000 times atmospheric pressure, however, the crystal structure changes so fundamentally that beryllium atoms gain a fifth neighbour. Meanwhile, an atmospheric pressure 880,000 times that at sea-level generates new structural changes that give rise to even a sixth neighbour.
"Although there are currently no technological applications for the new crystals, they are broadening the horizons of materials science. They show us that no irrevocable chemical certainties can be derived from normal conditions on the earth's surface. Extreme conditions and rare phenomena, which we can only create and observe in the laboratory using sophisticated technology, are actually normal in many places in the universe," says Prof. Dr. Leonid Dubrovinsky.
More information: Anna Pakhomova et al. Penta- and hexa-coordinated beryllium and phosphorus in high-pressure modifications of CaBe2P2O8, Nature Communications (2019). DOI: 10.1038/s41467-019-10589-z
Journal information: Nature Communications
Provided by University of Bayreuth