This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researcher uses hydrostatic pressure to understand RNA dynamics
Just as space holds infinite mysteries, when we zoom in at the level of biomolecules (one trillion times smaller than a meter), there is still so much to learn.
Rensselaer Polytechnic Institute's Catherine Royer is dedicated to understanding the conformational landscapes of biomolecules and how they modulate cell function. When biomolecules receive certain inputs, it can cause the atoms to rearrange and the biomolecule to change shape. This change in shape affects their function in cells, so understanding conformational dynamics is critical for drug development.
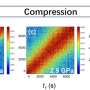
In research recently published in the Proceedings of the National Academy of Sciences, Royer and her team examined the conformational dynamics of a human transfer ribonucleic acid (tRNA) under high hydrostatic pressure. The high pressure led to an increased population of the tRNA-excited states that normally exist at very low levels, allowing new insights into tRNA function.
"We're interested in observing the excited states because they lead to conformations outside of those that can be determined by X-ray crystallography, nuclear magnetic resonance (NMR), or electron microscopy," said Royer. "We're beginning to understand that there are far more biomolecular structures than previously thought and, for the development of therapeutics, we need to understand what these states look like."
For this research, Royer used human tRNA rather than proteins, which are what she typically studies. "There hasn't been much work done on excited states of large RNA molecules, so that's what makes this research unique," Royer said.
Royer and team learned that the excited states not only play a role in the normal function of tRNAs for protein translation from the messenger RNA, but likely also play a role in HIV infection. HIV newly infects about 1.5 million people worldwide each year.
"The NMR revealed that the hydrogen bonds holding the tRNA together are weakened in these excited states," said Royer. "The small-angle X-ray scattering at high pressure, which we did at CHESS, revealed that the shape of the tRNA changed in these excited states. The areas that were altered by pressure also happen to be the areas that get hijacked by HIV during infection." CHESS, or the Cornell High Energy Synchrotron Source, is a state-of-the-art synchrotron radiation facility and the only one in the U.S. that enables high-pressure small-angle X-ray scattering (SAXS) measurements on biomolecules.
Royer and her team surmise that the excited state configurations of the tRNA they observed under pressure could be exploited by the invading viral RNA to initiate HIV reverse transcription. This process is linked to the virus's infectiousness.
"Dr. Royer's research, together with her team, may advance our understanding of how HIV spreads," said Deepak Vashishth, director of CBIS. "Further, over 80% of the microbial biomass on Earth exists at high pressure. Understanding how biomolecular sequences are adapted to function in high-pressure environments will yield new approaches for developing sturdier and more active biomolecules for biotechnology."
"It's an exciting time to be in high-pressure structural biology," said Richard Gillilan of CHESS. "People have known for some time that biomolecules do interesting things under extreme pressure, but, until very recently, technologies like high-pressure NMR and SAXS just weren't available to the general research community. Now, we can start to see what pressure does in molecular detail, and there is a lot of interest from multiple scientific fields, including biomedicine."
Royer was joined in research by Jinqiu Wang, Tejaswi Koduru , Balasubramanian Harish, Scott A. McCallum, , Karishma S. Patel, Edgar V. Peters, and George Makhatadze of Rensselaer; Kevin P. Larsen, Elisabetta V. Puglisi, and Joseph D. Puglisi of Stanford University; and Gillilan.
More information: Jinqiu Wang et al, Pressure pushes tRNALys3 into excited conformational states, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2215556120
Journal information: Proceedings of the National Academy of Sciences
Provided by Rensselaer Polytechnic Institute