This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Catalysis under the microscope is more complex than expected, shows new study
Catalysts composed from tiny metal particles play an important role in many areas of technology—from fuel cells to production of synthetic fuels for energy storage. The exact behavior of catalysts depends, however, on many fine details and their interplay is often difficult to understand. Even when preparing exactly the same catalyst twice, it often occurs that these two will differ in minute aspects and therefore behave very different chemically.
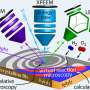
At TU Wien, scientists try to identify reasons for such effects by imaging the catalytic reactions taking place on various locations on these catalysts, applying several different microscopy techniques. Such an approach yields a reliable, microscopically correct understanding of the catalytic processes.
In doing so, it appeared that even relatively "simple" catalytic systems were more complex than expected. For example, it is not only the size of the employed metal particles or the chemical nature of the support material that define the catalytic properties. Even within a single metal particle, different scenarios can prevail on the micrometer scale. In combination with numeric simulations, the behavior of different catalysts could then be explained and correctly predicted.
Not all particles are the same
"We investigate the combustion of the possible future energy carrier hydrogen with oxygen, forming pure water, by using rhodium particles as catalysts," explains Prof. Günther Rupprechter from the Institute of Materials Chemistry at TU Wien. Various parameters play an important role in this process: How big are the individual rhodium particles? Which support material do they bind to? At which temperature and which reactant pressures does the reaction take place?
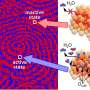
"The catalyst is made from supported rhodium particles, but it does not behave like a uniform object which can be described by a few simple parameters, as often tried in the past," highlights Günther Rupprechter. "It soon became clear, that the catalytic behavior strongly varies at different catalyst locations. A given area on a given rhodium particle may be catalytically active, whereas another one, just micrometers away, maybe catalytically inactive. And a few minutes later, the situation may even have reversed."
Nine catalysts at one sweep
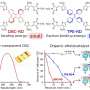
For the experiments, Dr. Philipp Winkler, the first author of the study published in the journal ACS Catalysis, prepared a stunning catalyst sample, comprising nine different catalysts with differently sized metal particles and varying support materials. In a dedicated apparatus, all catalysts could therefore be observed and compared simultaneously in a single experiment.
"With our microscopes, we can determine if the catalyst is catalytically active, it´s chemical composition and electronic properties—and this for each and every individual spot on the sample," says Winkler. "In contrast, traditional methods usually just measure an average value for the entire sample. However, as we have demonstrated, this is often by far not sufficient."
Even more complex than anticipated
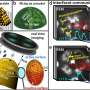
Chemical analysis on the microscopic scale has shown that the catalyst composition can vary locally even more than expected: Even within the individual metal particles strong differences were observed. "Atoms of the support material can migrate onto or in the particles, or even form surface alloys," states Rupprechter. "At some point, there is even no clear boundary anymore, but rather a continuous transition between catalyst particle and support material. It is crucial to consider this fact—because it also affects the chemical activity."
In a next step, the team at TU Wien will apply the gained insights and the successful methods to tackle even more complex catalytic processes, in their continuing mission to explain processes on a microscopic scale, to contribute to the development of improved catalysts, and to search for new catalysts.
More information: Philipp Winkler et al, Imaging Interface and Particle Size Effects by In Situ Correlative Microscopy of a Catalytic Reaction, ACS Catalysis (2023). DOI: 10.1021/acscatal.3c00060
Journal information: ACS Catalysis
Provided by Vienna University of Technology