This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Tiny proteins found across the animal kingdom may play a key role in cancer spread
Phosphatases of regenerating liver (PRLs) are a family of enigmatic proteins involved in cell growth and metabolism present in various species. From humans to fruit flies, they play a unique role in the growth of cancerous tumors and the spread of cancer throughout the body. New research emerging from McGill University is contributing to what is known about PRLs, which could potentially become an important tool in the development of cancer-fighting treatments.
Led by Kalle Gehring, a professor in the Department of Biochemistry and founding director of the McGill Centre for Structural Biology, the researchers focused on unraveling the mystery around PRLs. "It's important for us to study PRLs because they are so important in cancer," said Gehring, "In some cancers such as metastatic colorectal cancer, the proteins are overexpressed up to 300-fold."
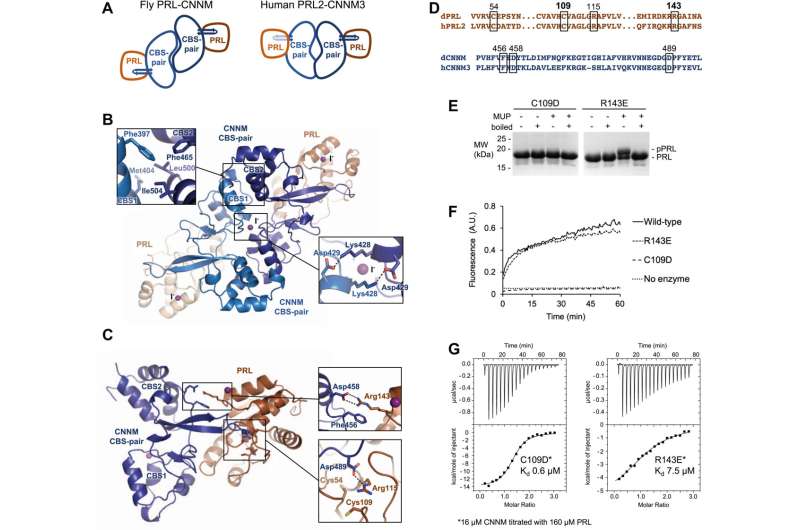
Published in the Journal of Biological Chemistry, Prof. Gehring and his colleagues (with data collected at the Canadian Light Source (CLS) at the University of Saskatchewan) confirmed that not only PRLs exist in all kinds of single- and multi-cell animals, but that the role of PRLs in binding magnesium transporters is common among all studied species.
This overexpression of PRLs makes cancer cells more metastatic and drives the spread to other organs. This data could help to further the understanding of how these proteins influence human disease.
"What we learned is that they all bind the magnesium transporters in the same way," says Gehring. "We're excited because it helps us understand this pathway, and that will reveal new targets for drugs to prevent cancer progression."
More information: Rayan Fakih et al, Burst kinetics and CNNM binding are evolutionarily conserved properties of phosphatases of regenerating liver, Journal of Biological Chemistry (2023). DOI: 10.1016/j.jbc.2023.103055
Journal information: Journal of Biological Chemistry
Provided by McGill University