This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Novel sustainable electrochemical method converts carbon dioxide into carbonaceous materials
Carbon dioxide (CO2) is a major greenhouse gas emitted through various types of human activities. In an effort to decrease humanity's carbon footprint, scientists and policymakers across the globe are continuously trying to explore new methods for reducing atmospheric CO2 emissions and converting them into useful forms. In this regard, the electrochemical method of reducing CO2 to other carbonaceous forms like carbon monoxide, alcohols and hydrocarbon has gained considerable attention.
Against this backdrop, environmental researchers from Doshisha University, Japan led by Prof. Takuya Goto recently published a study in Electrochimica Acta that demonstrated one such method for converting CO2 into multi-walled carbon nanotubes (MWCNT) using molten salts through sustainable electrochemistry. Their study was made available online, and included contributions from Dr. Yuta Suzuki from the Harris Science Research Institute and Mr. Tsubasa Takeda from the Department of Science of Environment and Mathematical Modeling.
Using a sustainable electrochemical technique, the research team facilitated the conversion of CO2 into MWCNT using LiCl-KCl melt. The molten salts were saturated with CO2 gas and semi-immersed nickel (Ni) substrate was used as electrode. The supplied CO2 was electrochemically converted to solid carbon at the end of the procedure. This green conversion occurred via a reduction reaction at the Ni electrode/LiCl-KCl melt/CO2 interface.
"The electrochemical reduction of CO2 on a Ni electrode in LiCl-KCl melt at 723K was studied. Under high polarization, a super meniscus was formed at the three-phase interface of the Ni electrode/LiCl-KCl melt/CO2 gas, where the direct electrochemical reduction of CO2 to solid carbon progressed. Solid carbon was obtained in the wetted area of the Ni electrode as well as in the bulk molten salt via the electrochemical technique," says Prof. Goto.
Subsequent characterization of the electrode-deposited carbon using electron microscopy techniques and elemental analysis revealed that the obtained carbonaceous material consisted of MWCNTs, commercially viable nanostructures, that were 30–50 nm in diameter. The team then varied the applied voltage and extended the reaction time, recording noticeable changes in the MWCNTs. The height of the generated MWCNTs increased after the electrolysis time was increased from 10 min to 180 min.
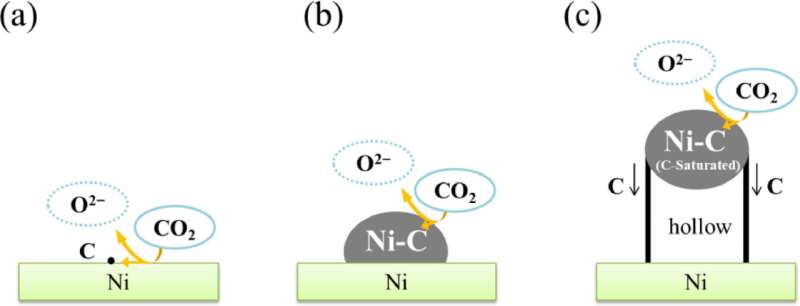
"We studied the dependence of applied potential and electrolytic time on the morphology and crystallinity of the electrodeposited carbon. Based on our experimental results, we proposed a model for the formation of the MWCNTs on the Ni electrode," says Prof. Goto.
The proposed model for the generation of MWCNTs from CO2 is described in three stages. The first stage involves the reduction of CO2 to carbon atoms at the Ni/LiCl-KCl melt/CO2 interface. During the second stage, the electrodeposited carbon atoms form Ni-C compounds (like NiC) on the surface of Ni electrode. Finally, when solubility limit of carbon in Ni-C compounds is reached, the cylindrical-shaped MWCNTs grow from the edge of the Ni-C compounds generated during the second stage.
In summary, the study identifies a novel process for sustainably converting CO2 into commercially useful carbonaceous materials. Moreover, the employed electrochemical process is environment friendly owing to the non-usage of fossil fuel. In addition, the use of high-temperature molten salts is unique because it enables the direct conversion of CO2 gas into MWCNTs.
"Our results indicate that CO2 can be converted into carbonaceous functional materials. By combining non-consumable oxygen-evolving anodes, this technique can contribute to the development of a carbon recycling technology that will not only solve global environmental problems but also play an important in carbon pricing economies. The material production process, which does not use fossil fuels, will help realize a sustainable society in the near future," says Prof. Goto.
More information: Yuta Suzuki et al, Direct electrochemical formation of carbonaceous material from CO2 in LiCl-KCl melt, Electrochimica Acta (2023). DOI: 10.1016/j.electacta.2023.142464
Provided by Doshisha University