This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
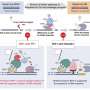
Mechanisms for removal of strong, replication-blocking lesions generated by the human HMCES protein

Researchers at Nagoya University and Osaka University in Japan have found novel repair pathways of apurinic/apyrimidinic (AP) sites of DNA. Repair of the base excision, which repairs AP sites, is an essential mechanism for cell survival. Its dysfunction causes genome instability disorders, including various cranial nerve diseases. The findings of this study, published in Nucleic Acids Research, should lead to a better understanding of the molecular mechanisms to repair AP sites that are the causes of unexplained and intractable genomic instability diseases.
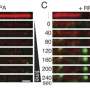
Recently, it was discovered that the HMCES protein prevents DNA cleavage by forming the DNA-protein crosslink with the AP site and that the DNA-HMCES crosslink protects cells from the toxicity of the AP sites. However, the mechanism by which the DNA-HMCES crosslinks when secondary DNA damage is repaired has yet to be revealed. In this study, the research team determined the repair mechanisms of DNA-HMCES crosslink damage.
This research is important because endogenous DNA damage induced by intracellular metabolites causes aging and carcinogenesis. One of the most frequently generated endogenous DNA damages is the AP site. Although AP sites in double-stranded DNA are repaired by base excision repair, human tissues accumulate between 50,000 and 200,000 AP sites per single cell.
The AP site is a site in which genetic information is lost and is susceptible to DNA strand breakage through a chemically unstable structure. During DNA replication, the exposed AP site on the single strand of the template DNA impedes the progress of DNA polymerases because of the loss of genetic information. It also causes serious DNA double-strand break due to AP site breakage, which would induce cell death.
More information: Yohei Sugimoto et al, Novel mechanisms for the removal of strong replication-blocking HMCES- and thiazolidine-DNA adducts in humans, Nucleic Acids Research (2023). DOI: 10.1093/nar/gkad246
Journal information: Nucleic Acids Research
Provided by Nagoya University