This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Cheese experiments show fungal antibiotics can influence microbiome development
Fungi produce metabolites that humans have used to improve health. For example, they secrete penicillin, which is then purified and used as an antibiotic for humans, leading to the development of many other antibiotics. However, the ecology of fungal metabolites in microbial communities is not well understood. In a new study, researchers use cheese rinds to demonstrate that fungal antibiotics can influence how microbiomes develop. The study is published in mBio.
"My lab is interested in how fungi shape the diversity of microbial communities where they live. Fungi are widespread in many microbial ecosystems, from soils to our own bodies, but we know much less about their diversity and roles in microbiomes compared to more widely studied bacteria," said principal investigator of the new study Benjamin Wolfe, Ph.D., associate professor in the Department of Biology at Tufts University.
His lab studies how fungi interact with other microbes in microbial communities, with a focus on fungal bacterial interactions. "To study the ecology of fungi and their interactions with bacteria, we use cheese rinds as a model microbial ecosystem to understand these basic biology questions," said Wolfe.
Cheese rinds are microbial communities that form on the surfaces of naturally aged cheeses such as Brie, Taleggio and some Cheddars. The fuzzy and sometimes sticky layers on the surfaces of these cheeses are communities of microbes that develop as the cheese is aged. They slowly decompose the cheese curd as they grow on the surface and produce aromas and pigments that give each artisan cheese unique properties.
Several years ago, a cheesemaker reached out to Wolfe with a mold problem: A mold was becoming abundant on the surfaces of the cheesemaker's cheeses and was disrupting the normal development of their rind. It appeared as though the rinds were disappearing as the mold invaded their cheese cave. This mold invasion provided a perfect opportunity for Wolfe and his colleagues to study the ecology, genetics and chemistry of fungal-bacterial interactions.
Wolfe's team began a collaboration with Nancy Keller's lab at the University of Wisconsin to try to figure out how this mold was impacting the rind microbial community. They wanted to find out what the mold was doing to the rind microbes and what chemicals the mold may be producing that could disrupt the rind.
To conduct their study, the researchers first deleted a gene (laeA) in the Penicillium mold that is known to control the expression of chemicals that fungi can secrete into their environment. These compounds are called specialized or secondary metabolites.
"We know that many fungi can produce metabolites that are antibiotics because we have used these as drugs for humans, but we know surprisingly little about how fungal antibiotics work in nature," said Dr. Wolfe.
"Do fungi actually use these compounds to kill other microbes? How do these antibiotics produced by fungi affect the development of bacterial communities? We added our normal and our laeA-deleted Penicillium to a community of cheese rind bacteria to see whether deleting laeA caused changes in how the community of bacteria developed."
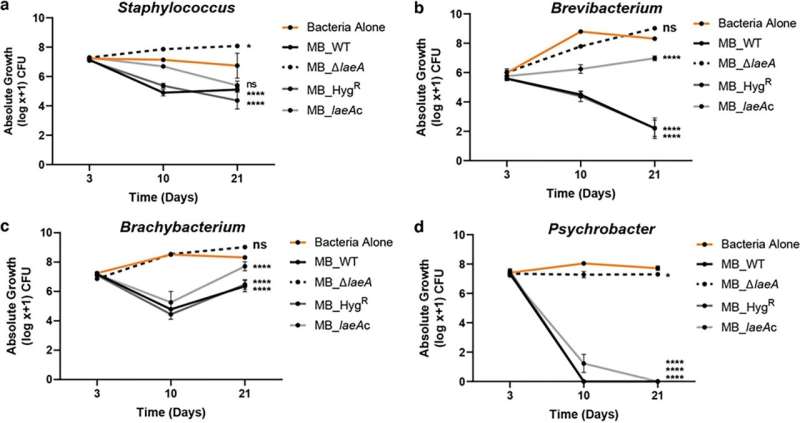
The researchers found that when they deleted laeA, most of the antibacterial activity of the Penicillium mold was lost. This was exciting because it allowed the researchers to narrow down specific regions of the fungal genome that might be responsible for producing the antibacterial compounds. They were eventually able to narrow it down to one class of compounds called pseurotins.
These are metabolites produced by a range of fungi that have been shown to have interesting biological activities including immune system modulation, insect killing and bacterial inhibition.
The study is the first to show that pseurotins can control how bacterial communities living with that fungi grow and develop. The pseurotins produced by the Penicillium mold in cheese are strongly antibacterial and dramatically inhibited certain bacteria compared to others (the bacteria inhibited were Staphylococcus, Brevibacterium, Brachybacterium, and Psychrobacter, which are found on many artisanal cheeses). This caused a dramatic shift in the composition of the cheese rind microbiome in the presence of the Penicillium-produced pseurotins.
This study demonstrates that antibiotics secreted by fungi can control how microbiomes develop. Because many fungi produce similar metabolites in a range of other ecosystems, from the human microbiome to soil ecosystems, the researchers expect that these mechanisms of fungal-bacterial interactions are widespread.
"Our results suggest that some pesky mold species in artisan cheeses may disrupt normal cheese development by deploying antibiotics," said Wolfe. "These findings allow us to work with cheesemakers to identify which molds are the bad ones and how to manage them in their cheese caves. It also helps us appreciate that every time we eat artisan cheese, we are consuming the metabolites that microbes use to compete and cooperate in communities."
More information: Joanna Tannous et al, LaeA-Regulated Fungal Traits Mediate Bacterial Community Assembly, mBio (2023). DOI: 10.1128/mbio.00769-23
Journal information: mBio
Provided by American Society for Microbiology