This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New technology more efficiently removes heavy metals from water

As freshwater scarcity affects millions worldwide, scientists and engineers have looked for new ways of filtering unwanted metals and minerals out of water while retaining those elements for re-use elsewhere.
Capacitive deionization (CDI), a technology in which a membrane made from electrode materials removes metal ions from water, has proved a promising technique for such next-generation water filters. Researchers from University of Chicago and Argonne National Laboratory envisioned the technique could be made even more efficient if they modified the molecular surface of the electrodes.
With support from University of Chicago's Joint Task Force Initiative, three researchers investigated the best way to alter these surfaces. Junhong Chen, Crown Family Professor of Molecular Engineering at UChicago's Pritzker School of Molecular Engineering and Lead Water Strategist at Argonne, collaborated with two Argonne colleagues: scientist Maria Chan and senior physicist Chris Benmore. Using experimentation, machine learning, and powerful X-rays, they developed a CDI device that adsorbed lead much more efficiently than before.
"Our future economy and national security really depend on the availability of clean water," Chen said. "The only way to really address this water challenge is to look into the re-use of water. This could potentially drive the recovery of resources that are important for clean energy applications and move us toward a circle economy for our society."
Finding the best functional molecular groups
Creating such a device could have wide-ranging implications. It could help remove lead from water to create safer drinking water, and it could help with the re-use of water by trapping phosphorus and lithium from a water supply, then releasing the phosphorus for use in fertilizer and the lithium for use in clean energy technologies.
Current technologies don't have the ability to selectively separate trace amounts of different ions within water, or can only do so at high cost. While some technologies can separate metals, they cannot fully distinguish one kind of metal ion from another. This is important, since while lead should be removed from drinking water, another metal, calcium, should be left in water, since it is beneficial for human health.
CDIs have shown promise in removing selected metal ions by using carbon materials like graphene oxide in their electrodes. To improve these devices, the UChicago-Argonne team needed to better understand the interaction between molecules and ions at the surface of the device's electrodes.
Using modeling and machine learning, Chan and her team worked to understand how altering functional molecular groups—specific groups of atoms that have their own characteristics—on the electrode surface would affect the selectivity and removal of metal ions.
"Using computer models, we can understand and select functional groups at a molecular level. Using machine learning models, and we can broaden the search to a number of different molecules that are potentially viable," Chan said.
The team ultimately found functional groups that could attach to the graphene oxide as well as selectively adsorb different types of ions from the water. Their predictions were experimentally validated by Chen, showing that the device could much more efficiently remove lead from water with these new functional groups.
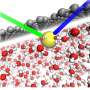
At Argonne, Benmore used the powerful Advanced Photon Source, a high-energy X-ray, to understand the structure of lead in water samples and better understand the interaction between the CDI and the lead. "These were based on experiments done back in the 80s, and no one has really studied ions in water since, so we were really able to pick up on a lot of very old but important work and really apply new methods towards it," he said.
Next the team plans to test its device using other metal ions, including lithium and cobalt. The project benefitted from early funding from UChicago Joint Task Force Initiative, which helps Argonne and Fermilab achieve mission success by opening channels of frequent communication and collaboration across institutions.
"The UChicago Joint Task Force Initiative support has been critical for this research project," Chen said. "I think this project has fostered collaboration between UChicago and Argonne researchers, which is really very impactful because we are going to work together for this project as well as future projects."
Provided by University of Chicago