April 27, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Removing the sluggishness associated with using hydrogen to make steel
A team of metallurgists and materials scientists at the Max-Planck-Institut für Eisenforschung GmbH has uncovered the reason for the sluggishness that occurs when attempting to use hydrogen instead of coke to make steel. In their study, reported in the journal Physical Review Letters, the group isolated the problem and offer solutions for producing steel with reduced carbon emissions.
Prior research has shown that making steel accounts for approximately 7% to 9% of carbon dioxide emissions into the atmosphere, which has led scientists to look for cleaner ways of making the material. Thus far, the prime approach has been using hydrogen to heat the iron oxide. But this has proven to be too sluggish. In this new effort, the research team has found the reason for the sluggishness and has also found a solution.
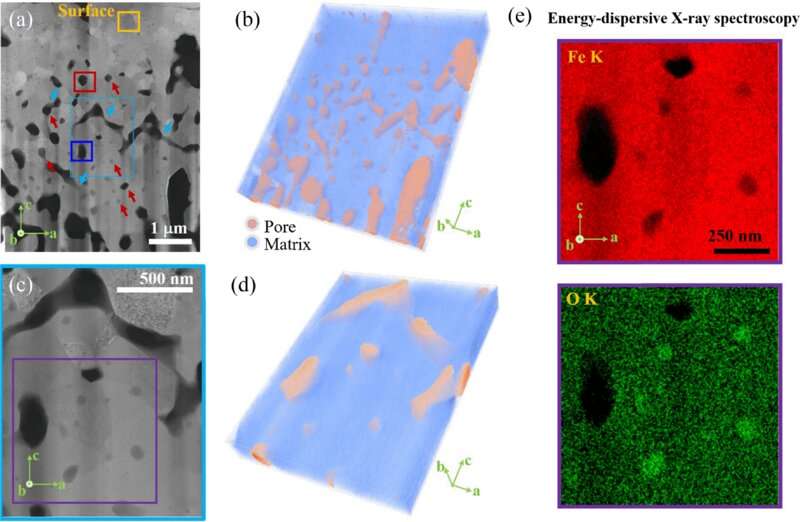
To make steel, coke (which has a high carbon content) is burned inside of a blast furnace to heat up a quantity of iron oxide. As this happens, a reaction occurs between the carbon and the oxygen in the iron, resulting in the release of carbon dioxide into the air as the iron is converted to steel. Prior research has shown that as the oxygen leaves the iron during the reaction, pores remain in the iron which must then be cleared of oxygen by heating the metal a second time. In this new effort, the researchers found that these pores led to problems when attempting to use the much cleaner hydrogen to heat the iron oxide.
By using an electron microscope, the research team was able to see that when hydrogen was used to heat iron oxide, the pores that were left behind in the reactions became filled with water, which subsequently became trapped inside the iron. That water then reoxidized the iron, which was slowing the oxygen removal process. They also found that sometimes, channels formed that allowed the water to drain. Thus, the solution to removing the sluggishness from the process was to heat the iron oxide in a way that promoted the creation of channels, which could be done by introducing a microfracture structure into the feedstock.
More information: Xuyang Zhou et al, Effect of Pore Formation on Redox-Driven Phase Transformation, Physical Review Letters (2023). DOI: 10.1103/PhysRevLett.130.168001
Journal information: Physical Review Letters
© 2023 Science X Network