This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists identify new benchmark for freezing point of water at -70 C
Scientists have discovered yet another amazing aspect of the weird and wonderful behavior of water—this time when subjected to nanoscale confinement at sub-zero temperatures.
The finding that a crystalline substance can readily give up water at temperatures as low as -70 °C, published in the journal Nature on April 12, has major implications for the development of materials designed to extract water from the atmosphere.
A team of supramolecular chemists at Stellenbosch University (SU), consisting of Dr. Alan Eaby, Prof. Catharine Esterhuysen and Prof. Len Barbour, made this discovery while trying to understand the peculiar behavior of a type of crystal that first piqued their interest about ten years ago.
"Scientists are currently adept at designing materials that can absorb water," Barbour explains. "However, it is much harder to get those materials (we call them 'hydrates') to then release the water without having to supply energy in the form of heat. As we all know, energy is expensive and seldom completely 'green.'"
The chemical compound in question was originally synthesized by Prof. Marcin Kwit, a specialist in organic stereochemistry at Adam Mickiewicz University in Poland. It was then crystallized and brought to Barbour's lab for further study by postdoctoral fellow Dr. Agnieszka Janiak. This was mainly because of Barbour's interest in ring-shaped molecules and how they form channels when packed together in crystals.
Janiak noticed that the crystals were yellow on some days and red on others. It didn't take her long to figure out that the crystals would only turn red on days with humidity levels higher than 55%. When humidity levels fell below this level, the crystals would go back to being yellow.
"Not only was this behavior rather unusual," Barbour explains, "it was also happening very fast. It seems the crystals were absorbing water as fast at high humidity as it was losing it again at low humidity. While we are familiar with materials designed to absorb water, it is highly unusual for a material that absorbs water easily to lose it equally easily."
Why do these crystals have such special properties? This question started a nearly ten-year investigation, which initially focused on explaining the mechanism behind the color change. Theoretical modeling by Esterhuysen and MSc student Dirkie Myburgh showed that water uptake causes slight changes in the electronic properties of the crystals, causing them to turn red. With such remarkable properties, Barbour was convinced that the crystals would also have other interesting properties.
That is when Ph.D. student Alan Eaby started dabbling with the material. Initially he had focused on room temperature studies for his MSc research but would later turn his attention to measuring properties at lower temperatures when he embarked on his Ph.D. three years ago. He wanted to know how the crystals would behave when subjected to different temperatures and humidity levels: "I was intrigued by the color change and wanted to explore what was happening at the atomic scale," he explains.
Having learned about developing instruments and methods from Barbour, he embarked on employing non-standard techniques to understand the mechanisms of water uptake and release in the material.
One day, he observed something strange happening at temperatures below zero degrees Celsius. "I noticed that the crystal still changed color at sub-zero temperatures. Initially I thought that there was something wrong with the experimental setup or the temperature controller, as crystal hydrates are not supposed to release water at such low temperatures," he explains.
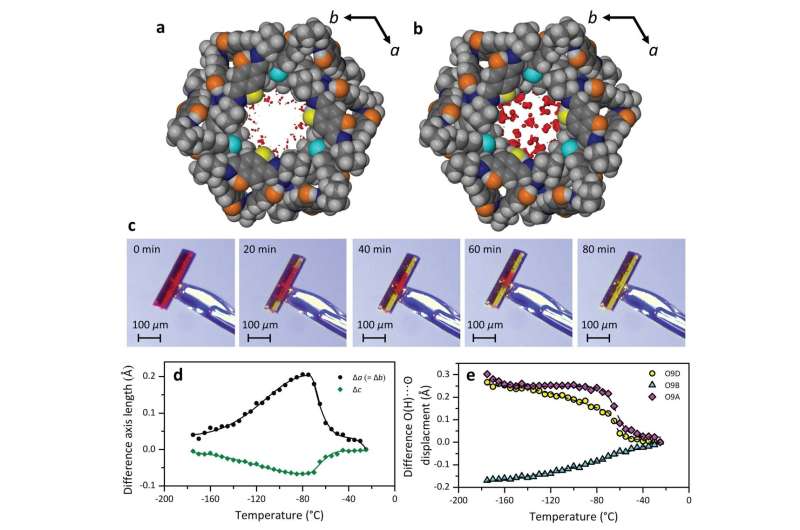
After lots of conversations and coffee breaks with Barbour and Esterhuysen, and tweaking the experimental setup several times, they realized that Alan's observations could be explained by the narrowness of the channels in the material. The channels in the crystal are only one nanometer wide—one thousandth the diameter of a human hair.
It was already known that, at the nanoscale, water can remain mobile within channels at temperatures below 0°C. However, this study showed for the first time that such channels can also allow the uptake and release of water at temperatures far below its normal freezing point.
To understand this process, Eaby undertook an extensive, systematic series of X-ray diffraction studies of the red and yellow crystals at different temperatures and humidities. This allowed him to construct a computer-generated "movie," with atomic-scale resolution, of what happens to the channels upon cooling or heating, and in the presence or absence of water. These animations indicated that water molecules in the nanochannels move about freely until cooled to -70 °C, whereupon they undergo a "reversible structuring event" to resemble a glassy state. This "glass transition" ultimately causes the water to become trapped in the material at temperatures below -70°C.
Were it not for the color-changing behavior of the crystals in the first place, they would not have become aware of the ultralow temperature water loss capability. "Who knows," says Barbour, "there may be many other materials out there with the ability to absorb and release water at very low temperatures, such as metal-organic frameworks and covalent organic frameworks.
"We simply do not know about it because we have not been able to visualize it. Now that we do know that such behavior is possible, it opens a whole new field of research and potential applications. Researchers can use this new information to identify other materials with similar properties, and also use the principles we've developed to fine tune the low-temperatures release of water. This could lead to dramatic reductions in the energetic costs of atmospheric water harvesting, with implications for society and the environment," he concludes.
More information: Alan C. Eaby et al, Dehydration of a crystal hydrate at subglacial temperatures, Nature (2023). DOI: 10.1038/s41586-023-05749-7
Journal information: Nature
Provided by Stellenbosch University South Africa