This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Developing cells likely can 'change their mind' about their destiny
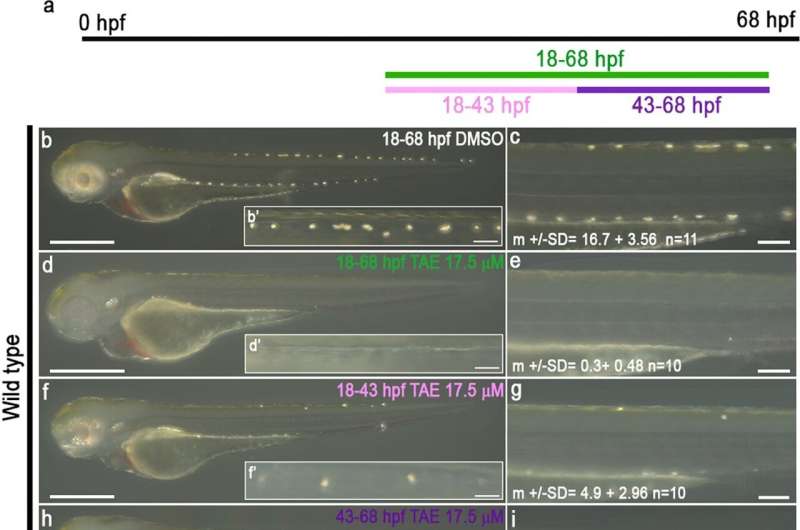
A neural crest cell (a type of stem cell) begins with the ability to differentiate into any number of specialist cell types, but it also appears to retain the capacity to "change its mind" and differentiate anew when the circumstances are right, according to new research from the University of Bath. As a result of this hyper-flexibility, the possibilities for these cells in replacing damaged human tissue is likely to be even greater than previously thought.
Neural crest cells—found in very young embryos, and vital for determining the color of hair and skin—are highly flexible by nature, giving rise to many different types of vital cells, including neurons. New research from the University of Bath suggests their flexibility remains greater than previously thought, a finding that has significant implications for regenerative medicine.
Until now, it was assumed that neural crest cells became committed to becoming a particular cell type very early, after which their fate was sealed. However, studies led by Professor Robert Kelsh from the Department of Life Sciences at Bath suggest they retain their adaptability even after they have become visibly differentiated.
This newly discovered flexibility helps explain why neural crest stem cells—an important type of stem cell that can also be readily isolated from adult skin—have immense potential as treatments to replace and repair damaged body tissue in many parts of the body.
The finding that even after choosing a destiny (for instance, developing into skin pigment cells), neural crest cells might be able to "change their mind" and choose a new destiny (perhaps becoming cartilage cells) reconciles a long-standing debate among biologists over the nature of neural crest cell differentiation.
Two competing theories
In humans, neural crest cells are multipotent, meaning they are capable of developing into many different types of cell, including cells of the peripheral nervous system, cardiac muscle, and cartilage, as well as pigment cells in the skin and hair. These are all cells with highly specific functions.
Until now, two rival theories have sought to explain how, exactly, they pull this off.
"The question of how the fate of these cells becomes decided and restricted has been unclear and much debated for over 40 years," said Professor Kelsh.
According to the first theory, neural crest cells begin to commit to a specific role in the young embryo before leaving the place from which they arise—the neural tube (which develops into the brain and spine). The thinking goes that by the time they start migrating to their final destination—be that the gut, skin, or connective tissue—their destiny is already partially limited (i.e., some options are already off the table) and that more and more options become eliminated as they migrate.
The second theory posits that neural crest cells remain multipotent when they leave the neural tube and only commit to a specific differentiation path once they reach their destination.
There has been a general feeling in the field that the first model was the more accurate of the two. The new study published in Nature Communications, however, finds that neither of these "static" theories is correct.
"It would appear that these cells are choosing their fate in a much more dynamic, mobile way and are not narrowing their options irreversibly until much later than we previously thought," said Professor Kelsh. "This provides experimental biologists with a new, updated model to help them understand the behavior of neural crest cells."
It has long been known that neural crest cells use molecular signals from their environments to turn into one type of cell or another. However, Professor Kelsh's genetic work on zebrafish—a freshwater fish with many genetic similarities to humans—shows these steps are likely reversible: remove the signals and the cells revert to a more primitive state, where their potential to differentiate differently is restored.
Professor Kelsh said, "Our work shows these cells become biased by their environment. Take them out of that environment and they relax back to a more broadly competent state, likely capable of becoming anything."
He added, "Our findings will be of interest to other stem-cell researchers, as they give us a theoretical understanding of how neural crest cells might be used in medicine to repair any number of defects, from skin-pigmentation defects such as vitiligo to defects of the nervous system."
More information: Tatiana Subkhankulova et al, Zebrafish pigment cells develop directly from persistent highly multipotent progenitors, Nature Communications (2023). DOI: 10.1038/s41467-023-36876-4
Journal information: Nature Communications
Provided by University of Bath