This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Protein deficiency in neurons of patients with neurodegenerative diseases could be targeted by new gene therapy approach

TDP-43 is an RNA-binding protein that normally resides in the nucleus of neurons but is abnormally located in the cytoplasm of neurons in most patients with amyotrophic lateral sclerosis (ALS) and frontotemporal dementia, and up to half of patients with Alzheimer's disease.
A team including investigators at Massachusetts General Hospital (MGH), a founding member of Mass General Brigham (MGB), previously showed that loss of nuclear TDP-43 leads to abnormalities in the RNA that encodes a protein essential for the ability of neurons to regenerate their axons after injury and to keep their connection with muscles to control movements.
In new research published in Science, the group has uncovered the details behind these deleterious effects and has developed an approach to fix them.
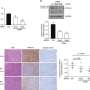
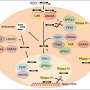
The protein that the investigators discovered to be most strongly affected by TDP-43 is called stathmin-2. The absence of nuclear TDP-43 leads to abnormal processing of stathmin-2 RNA, resulting in elevated levels of a non-functional truncated stathmin-2 RNA and a striking loss of stathmin-2 protein in neurons.
"Stathmin-2 disruption is a prominent abnormality observed in patients with a spectrum of neurodegenerative diseases, including almost all instances of sporadic and familial ALS, as well as a large portion of patients with dementia," says Clotilde Lagier-Tourenne, MD, Ph.D., an associate professor of Neurology at MGH and Harvard Medical School.
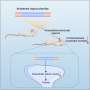
In this latest work, Lagier-Tourenne and her collaborators at the University of California San Diego and the Jackson Laboratory found that nuclear TDP-43 blocks certain sites in stathmin-2 RNA to protect them from being misprocessed (or in technical terms, "misspliced"). This protection, which is critical for the production of normal stathmin-2 protein, is absent when TDP-43 is aberrantly located in the cytoplasm.
To restore this protection, the researchers, in collaboration with IONIS Pharmaceuticals, designed antisense oligonucleotides (ASOs)—short, synthetic, single strands of genetic material that can bind to RNA—that were capable of suppressing abnormal splicing and boosting stathmin-2 protein levels in TDP-43–deficient human neurons. The ASOs essentially took over the role of nuclear TDP-43 by binding to and protecting sites in stathmin-2 RNA.
Finally, in mice that were gene-edited to contain abnormal stathmin-2 RNA, injection of the ASOs into the cerebral fluid (an approach currently used in the clinic for approved ASOs) corrected stathmin-2 RNA missplicing and restored stathmin-2 protein levels.
"Among the most promising translational strategies of gene therapy for neurodegenerative diseases, ASOs have emerged as a viable, life-extending therapeutic approach by correcting fatal gene expression or RNA processing defects. This work demonstrates the in vitro and in vivo efficacy of ASOs in preventing missplicing of stathmin-2 in TDP-43– deficient neurons," says Lagier-Tourenne.
"Our next steps are to further the clinical development of ASOs targeting stathmin-2 misprocessing in TDP-43 proteinopathies and to conduct additional investigations to better understand stathmin-2's role in neurons."
More information: Michael W. Baughn et al, Mechanism of STMN2 cryptic splice-polyadenylation and its correction for TDP-43 proteinopathies, Science (2023). DOI: 10.1126/science.abq5622
Journal information: Science
Provided by Massachusetts General Hospital