This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Understanding how cohesin makes DNA loops in the human genome and its role in Cornelia de Lange syndrome
Cohesin is a ring-shaped protein that surrounds and moves around the DNA molecule, forming the loops. It is a crucial process for the cell. Understanding how cohesin works has been one of the challenges of molecular biology in recent decades. A study now published by researcher Ana Losada's group at The Spanish National Cancer Research Centre (CNIO) will serve to deepen our understanding of the disease known as Cornelia de Lange syndrome.
At the end of the 1990s, Losada, who was then at the Cold Spring Harbor Laboratory (New York, U.S.), discovered in frogs of the Xenopus genus the protein cohesin, which is fundamental to the process of cell division.
Over the next few years, it became clear that cohesin performs not just one, but several crucial tasks for the cell, including folding the DNA into loops. The DNA inside each cell is several meters long (if measured linearly), so it has to be folded to fit into the cell nucleus; this folding is important in itself, because the spatial arrangement of DNA loops has been shown to affect how genetic information is decoded.
In short, the study of cohesin is now a very active area of research. Losada, head of the Chromosome Dynamics Group at CNIO, continues to lead the field. In her new work, she and her team elucidate a key point about how cohesin attaches to DNA and moves around the molecule forming the loops.
Their results, published in the journal Nature Communications, will further our understanding of the disease known as Cornelia de Lange syndrome.
One ring to organize the genome
A metaphor for visualizing cohesin in action might be that of a ring that traps two segments of a DNA strand to form a small loop, which becomes progressively longer as the ring moves. Cohesin is the ring.
"With its particular ring shape, this protein embraces the DNA, and in doing so it can do two jobs," explains Losada. "One is to hold together the two copies of each chromosome that the cell creates before cell division takes place, and the other is to facilitate the folding of the genome into loops of DNA, allowing these long strands to fit into a tiny container like the nucleus of a cell."
The cohesin binds to the DNA at a precise location on the chromosome, and without letting go, moves in a way that generates progressively longer loops, until it releases the DNA or an obstacle stops it and temporarily stabilizes it. These loops arrange the genetic material within the nucleus and facilitate communication between distant regions of the chromosome, for example genes and their regulatory elements.
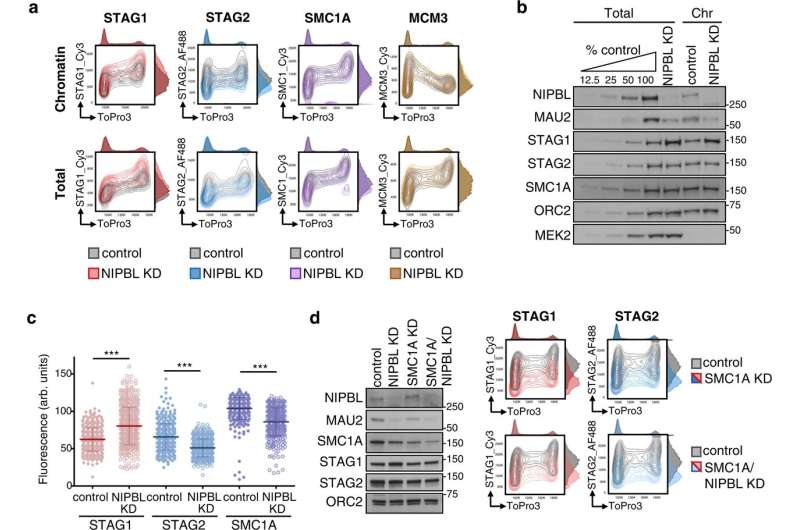
The new work focuses on a protein called NIPBL, which was previously thought to be necessary to attach cohesin to DNA and propel it as it moves through the genome. The CNIO team now believes that this is not how it works.
"There has been consensus among the research community that NIPBL is necessary to attach cohesin to DNA, and that it also helps it to move along the genome. Our results suggest that the former may not be true," says Dácil Alonso, first author of the paper.
The now-published paper shows that the NIPBL protein is not necessary for cohesin to bind to DNA, but only for it to move and form DNA loops.
Cohesin and Cornelia de Lange syndrome
This new model may be important in understanding Cornelia de Lange syndrome, a genetic disorder that affects one in 10,000 to 30,000 children born each year worldwide and causes severe physical and cognitive impairment.
In vertebrate cells, there are two versions of cohesin, known as STAG1 cohesin and STAG2 cohesin, although it is not known why. The CNIO group wondered whether the absence of NIPBL in the cell affects the two versions of cohesin in the same way, and discovered that it does not. By removing up to 85% of NIPBL in the cells, the STAG1 version was barely affected, while the STAG2 version almost disappeared from the genome.
Given that most patients with Cornelia de Lange syndrome have a mutated NIPBL gene, the new study "suggests that the cohesin most affected in them will be STAG2," explains Losada. "However, we are still a long way from understanding this complex disease."
More information: Dácil Alonso-Gil et al, Different NIPBL requirements of cohesin-STAG1 and cohesin-STAG2, Nature Communications (2023). DOI: 10.1038/s41467-023-36900-7
Journal information: Nature Communications
Provided by The Spanish National Cancer Research Centre