This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers propose titanium-based perovskite for water activation and lower-temperature hydrolysis of organic sulfur
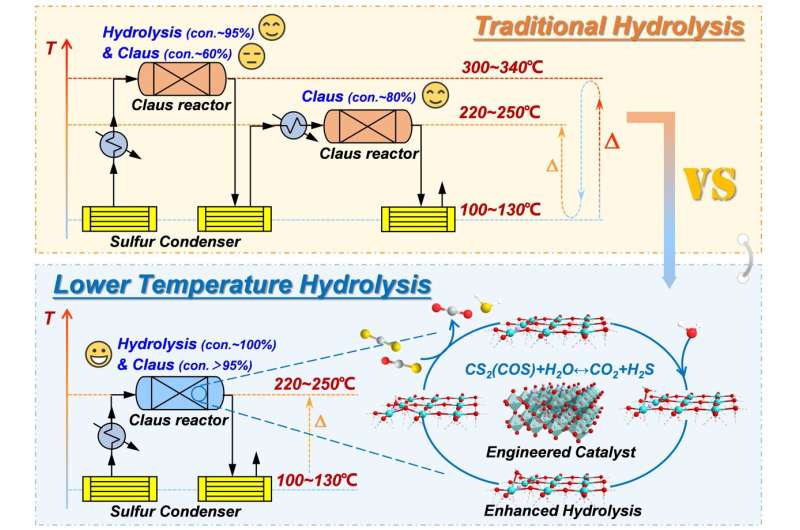
According to a study published in Proceedings of the National Academy of Sciences on Jan. 11, researchers led by Prof. Hao Zhengping from the University of Chinese Academy of Sciences (UCAS) have proposed facile oxygen vacancy (VO) engineering on titanium-based perovskite to motivate water (H2O) activation, achieving enhanced organic sulfur hydrolysis and efficient sulfur recovery at lower temperatures.
Water activation is involved in many reactions and processes. Organic sulfur (COS and CS2) hydrolysis is one such typical reaction using the H2O molecule as a reactant. In industrial sulfur recovery processes, an additional hydrolysis catalyst is installed in the first stage of the Claus reactor to convert COS and CS2. Limited by the effective activation of water, relatively higher temperatures are required for the hydrolysis of CS2 in a complex environment, which is not conducive to the sulfur recovery reaction, and thus severely affects the sulfur recovery efficiency and pollution emission control of the Claus process.
In this study, the researchers fabricated titanium-based perovskites with different VO contents, and found that there was a linear correlation between the VO content and the degree of H2O dissociation and hydrolysis performance. The low-coordinated Ti ions adjacent to VO were active sites for H2O activation.
The introduction of VO, especially VO clusters, resulted in the reduction of the energy barrier for H2O dissociation, which contributed to H2O activation and dissociation, according to the researchers.
Furthermore, their in-depth mechanism study revealed that CS2 hydrolysis was initiated from the reaction between dissociatively adsorbed -OH and gaseous CS2 (Eley-Rideal mechanism), which was the origin of the enhanced hydrolysis activity from the enhanced H2O activation by VO.
Consequently, complete conversion of COS and CS2 was achieved over SrTiO3 after H2 reduction treatment at 225°C, a favorable temperature for the Claus conversion. Surprisingly, the catalyst also showed excellent Claus catalytic activity.
Thus, both efficient organic sulfur hydrolysis performance and improved sulfur recovery efficiency can be achieved simultaneously. The application of this dual-functional catalyst can significantly improve the sulfur recovery efficiency, simplify the operation process and reduce the investment and operational cost.
Prof. Hao's team has long been engaged in the research and development of acid gas pollution control and resource recovery and utilization. They have an in-depth understanding of acid gas emission reduction and control. The production and publication of the results are based on the team's work over many years and are of great significance to the emission reduction control, resource recovery and utilization of acid gas.
More information: Zheng Wei et al, Oxygen vacancy-engineered titanium-based perovskite for boosting H 2 O activation and lower-temperature hydrolysis of organic sulfur, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2217148120
Journal information: Proceedings of the National Academy of Sciences
Provided by Chinese Academy of Sciences