This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How physics changes drug resistance evolution

A deeper understanding of how tumor cells respond to treatment is vital to improving the effectiveness of therapies for diseases such as cancer. Researchers at the Max Planck Institute for the Science of Light (MPL) have discovered how physical interactions between cells can allow treatment-resistant cells to survive in tumors, despite growing slower than non-resistant cells.
Mutations creating resistance to medication, are a major challenge to modern antibiotic and anti-cancer therapy. There is good news though: The same mutations that make, for example, cancer cells resistant to treatment, often put them at an evolutionary disadvantage compared to non-mutated cells. They pay for the ability to resist treatment with a decrease in growth rate.
This so-called fitness cost can cause a population of cells to be purified via natural selection, as the mutated cells grow slower and are thus outcompeted. However, these mutated cells can mutate again, compensating for their disadvantage in a so-called evolutionary rescue. How exactly these processes occur remains unclear due to the difficulty of tracking the mutated cells in space and time.
This is where a research team around MPL scientists Serhii Aif, Nico Appold and research group leader Jona Kayser steps in. In a new paper published in Nature Communications, authored together with Lucas Kampman and Oskar Hallatschek from the University of California, Berkeley, they used expanding yeast colonies augmented with fluorescence-coupled synthetic mutations to track the entire evolutionary trajectory of thousands of resistant lineages.
In doing so they gained new insights into the development of drug resistant mutant lineages of cells. Recent research results, by Jona Kayser among others, had already shown that in dense populations of cells, such as most forms of cancerous growths, the power of natural selection to weed out the slow-growing resistant mutants can be decreased by several orders of magnitude. Because the mutants are more likely to survive longer, they also have a higher chance of being rescued by a second mutation.
How exactly these effects play out was not previously known.
Mechanical interactions and natural selection in balance
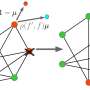
In the study, the team introduced a model system of radially growing 2D colonies comprised of genetically tailored yeast cells. This allowed them to study dense populations with control over variables such as rate of mutations and fitness cost of resistance. The slower-growing resistant yeast cells were augmented to be red-fluorescent and could mutate again at a rate controlled by the researchers, at which point they compensated for the fitness cost, lost their disadvantage and changed their fluorescence to cyan.
Using this system, Serhii Aif et. al. found the surprising result, that (as the populations expand) the mechanical interactions between the cells can act as a counterbalance to the evolutionary selection process. Normally natural selection would cause the mutated slow-growing cells to be outcompeted by the faster-growing unmutated cells.
Instead, the physical interactions can create a balance, which the researchers term inflation-selection balance, that allows resistant populations at the growing front (the outer edge of a population, where growth happens) of a colony to maintain their size at an equilibrium as the colony expands.
This then makes it more likely that the resistant cells mutate again. As these resistant cells with a second mutation start to spread, they can cause treatment to fail. At the same time, their effect on treatment failure is delayed. The red mutants survive longer because of the inflation-selection balance, but ultimately cannot grow to a size sufficient to survive random fluctuations, known as genetic drift.
By contrast, cyan mutants grow linearly with colony radius but need time to grow to a size at which random fluctuations do not wipe them out.This causes the delay between the mutations occuring and impacting treatment failure probability. Finally, using in silico tumor modeling, the team proved that this inflation-selection balance only requires a minimal set of conditions to be present. These conditions are inherent to many radially expanding dense populations and should therefore also be present in solid tumors.
Serhii Aif was surprised how the discovered balance allows once-mutated lineages to continue to survive as the colony expands. He says, "I believe we were able to discover and understand this balance process, because we approached it from a physics perspective, focusing our attention on the mechanical cell-cell interactions and the collective phenomena that emerged from it."
The published results are a new step in increasing our understanding of how resistances can develop during treatment of illnesses such as cancer. A deeper understanding here may lead to new and improved approaches to treatment in future. For the MPL group, the next step is trying to understand how they can use these insights from physics and combine them with deep reinforcement learning to improve evolution-based therapy strategies.
More information: Serhii Aif et al, Evolutionary rescue of resistant mutants is governed by a balance between radial expansion and selection in compact populations, Nature Communications (2022). DOI: 10.1038/s41467-022-35484-y
Journal information: Nature Communications
Provided by Max Planck Institute for the Science of Light