This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Converting drycleaning solvent into useful chemical compounds
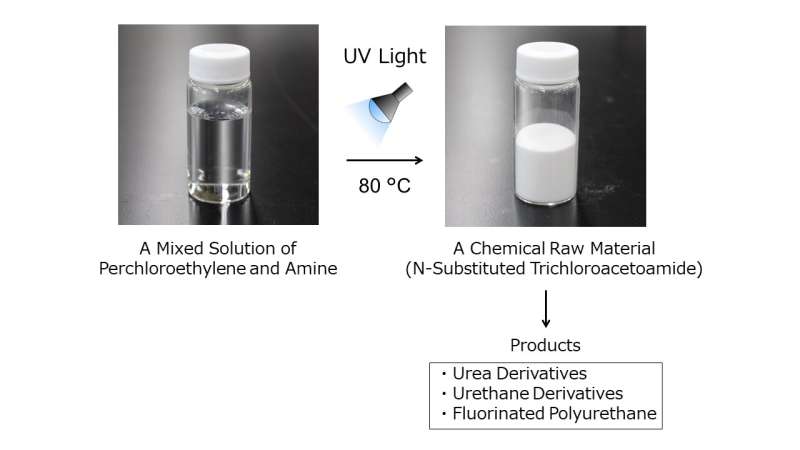
A collaboration between Associate Professor Tsuda Akihiko's research group at Kobe University's Graduate School of Science and AGC Incorporated has succeeded in synthesizing various useful compounds from perchloroethylene (also known as tetrachloroethylene), a solvent commonly used to dry clean clothes.
The compounds they synthesized include pharmaceutical intermediates (trichloroacetamide, urea derivatives and urethane derivatives) as well as a novel polyurethane containing a fluoroalkyl group. These useful chemical compounds are vital building blocks for manufacturing medicines, plastics and other products.
Utilizing Kobe University's previously invention: the 'photo-on-demand' organic synthesis method, the research collaboration developed a new photo-oxidation method (chemical reaction and process) for perchloroethylene. This simple, safe and low cost method places less burden on the environment and can efficiently synthesize the aforementioned useful compounds.
Perchloroethylene is already used in large quantities for dry cleaning and other applications. As the world moves towards carbon neutral efforts and sustainability, this research development has gained attention as a novel way of using perchloroethylene and as method for recycling chemicals.
Patents were filed in relation to these research findings in March 2019 (domestic) and March 2020 (international). The academic paper was published online in ACS Omega.
Main points
- Successful joint research collaboration combined the strengths of Kobe University, with its photo-on-demand synthesis method, and AGC Inc.'s experience as a producer of perchloroethylene.
- They developed a new organic synthesis method using perchloroethylene as raw material for the synthesis of chemical products. Perchloroethylene is commonly used for dry cleaning clothes (In 2018, Japan exported 6300 tons and imported 20 tons. Source: The Chemical Daily Co., Ltd.).
- The research group succeeded in synthesizing a ~97% yield of trichloroacetamide (a precursor for pharmaceuticals and polymers) by merely irradiating a mixture of perchloroethylene and a reactant (amine) with light. They managed to synthesize 21 chemicals on a scale of up to ten grams (and this can be scaled up).
- Using the above product, they successfully synthesized 11 pharmaceutical urea derivatives, 8 urethane derivatives and 1 fluorinated polyurethane on a gram scale.
- Expensive specialized apparatus and agents are not required. These useful chemical products can be synthesized in large quantities on demand using light in a safe, inexpensive and simple manner, which has a low impact on the environment.
- This could be utilized as a new method of recycling perchloroethylene.
- It is hoped that this new chemical synthesis method will greatly contribute towards efforts to become carbon neutral and realize sustainable societies.
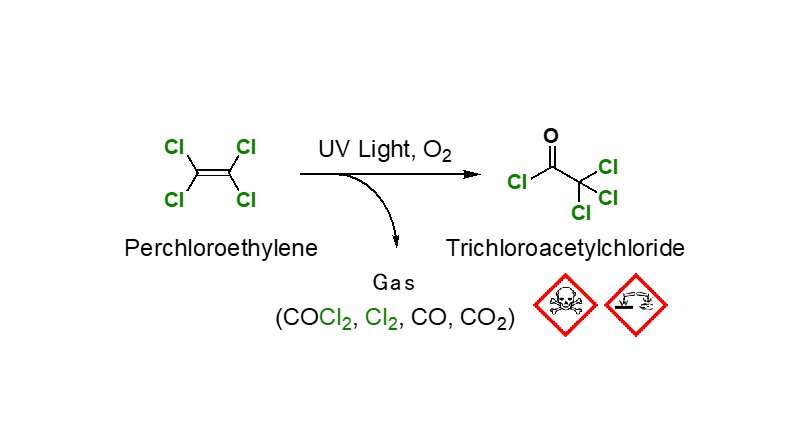
Perchloroethylene is mainly used as a dry cleaning solvent and metal degreaser. Even though perchloroethylene is inflammable and chemically highly stable, various substances are produced if it is photo-oxidized under ultraviolet light. These include trichloroacetyl chloride, phosgene, carbon monoxide and chlorine. These substances are highly damaging to the environment due to their extremely toxic or corrosive properties, however they are valuable raw materials for organic synthesis. In light of current environmental pollution issues, the development of a method to recycle perchloroethylene would be beneficial. Despite this, there are hardly any examples of using photo-oxidation products specifically from perchloroethylene for organic synthesis.
Associate Professor Tsuda et al.'s research group were global pioneers in this regard, successfully synthesizing numerous useful chemical compounds using perchloroethylene's photo-oxidation products. In 2012, they applied for a patent on this innovation. However, there were two remaining issues with this previous method, one scientific and one safety-related, respectively: 1. The reaction efficiency was low, and 2. It was necessary to temporarily take the toxic and corrosive photo-oxidation products out of the reaction chamber to use them to synthesize the target compound. To resolve these issues, this research group began working with AGC Inc. This research collaboration between industry and academia led to the joint development of an organic synthesis method that could safely, inexpensively and easily turn perchloroethylene into high yields of organic chemical compounds at low cost to the environment.
The researchers hoped that it would be possible to develop a novel organic synthesis method that resolved the previously mentioned safety issues. This would involve using a mixture of perchloroethylene and an amine, and would require an instant reaction to occur in situ between the perchloroethylene's photo-oxidation products and the amine upon direct irradiation with ultraviolet light. However, the researchers hypothesized that perchloroethylene photooxidation would not occur based on the common scientific knowledge that amines absorb ultraviolet light.
Even if the photo-oxidation did take place, they predicted that the subsequent reaction would not progress as hoped. This is because the hydrochloric acid (HCl) produced in the reaction between the photooxidation products and the amine would turn the amine into hydrochloride salt. In experiments under normal reaction conditions (below room temperature), the researchers found that the photooxidation of perchloroethylene proceeded slowly and the amine formed into hydrochloride salt at the same time.
A long period of exposure caused part of the amine to photodecompose, resulting in strong coloration. These experiments showed that perchloroethylene photooxidation results in a highly complex series of chemical reactions.
Therefore, the research group heated a mixed solution of perchloroethylene and amine to over 70°C in the hope that (1) the gas-phase reaction rate would be increased by partially vaporizing the perchloroethylene and (2) the reaction would proceed even if the amine formed into hydrochloride salt. The researchers found that the expected reaction proceeded in a short amount of time and a single product was obtained.
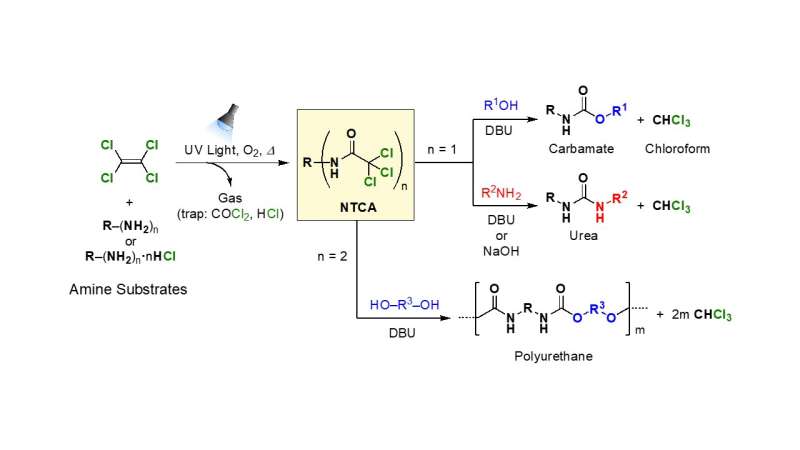
Based on this discovery, the researchers synthesized ~97% yields of various N-Substituted Trichloroacetamides (NTCAs, which chemically protect isocyanates that serve as urethane precursors). Therefore, it is thought that the mechanism behind perchloroethylene's photooxidation is not merely a photooxidation reaction.
Instead, it likely occurs as the result of a radical chain reaction caused by the chlorine radicals that are generated when the light cleaves the C-Cl bonds. The produced trichloroacetyl chloride instantly reacts in situ with amine and amine chloride, and this reaction is thought to be what causes NTCA to form.
The research group succeeded in synthesizing up to 10 grams of a total of 21 compounds including fluorinated ones, therefore this novel organic synthesis method can be applied to the production of a wide range of useful chemical compounds. Furthermore, it is possible to scale up the method merely by using larger reaction chamber.
The obtained NTCAs can be converted into urea derivatives and urethane derivatives through base-catalyzed substitution reactions with amines or alcohols. These reactions also produce chloroform as a by-product (which could be used as a recycled solvent or as a chemical precursor).
It is thought that NTCA decomposes into isocyanates and then the subsequent addition of amines or alcohols progresses the reaction. In one application of this chemical reaction, the researchers successfully synthesized a new fluoroalkyl polyurethane from an NTCA containing fluorine. Fluoroalkyl compounds tend to have water repellent, oil repellent, flame retardant and weather resistant properties.
Further research
Using the photo-on-demand synthesis method, the research group were able to synthesize various organic chemical compounds (including urea derivatives, urethane derivatives and polyurethane) from a solution of perchloroethylene and amine in a safe, inexpensive and relatively environmentally friendly manner.
To develop this photoreaction method even further, Professor Tsuda et al. are currently using a flow reaction system in their efforts to develop a continuous organic synthesis method. With its potential to contribute towards the realization of a sustainable society, this research accomplishment is expected to be utilized in the future as a novel way of using the abundant chemical perchloroethylene and as a method to recycle chemicals.
More information: Toshiki Akamatsu et al, Photo-on-Demand In Situ Synthesis of N-Substituted Trichloroacetamides with Tetrachloroethylene and Their Conversions to Ureas, Carbamates, and Polyurethanes, ACS Omega (2023). DOI: 10.1021/acsomega.2c07233
Journal information: ACS Omega
Provided by Kobe University