A more sustainable version of 'click chemistry'
20 years ago, Danish chemist Morten Meldal discovered a new method for assembling molecules.
Assembling molecules has always been and still is a large part of chemists' work—whether they are hired to develop new sewage treatment plants, sunscreens, batteries or dishwashing liquid; no new product is created until the molecules are put together correctly.
Meldal's discovery was called click chemistry, and this year it won him the Nobel Prize in Chemistry (along with Barry Sharpless and Carolyn Bertozzi).
"Click chemistry is a huge discovery, and the Nobel Prize is fully deserved," says Changzhu Wu, who is a chemist and an associate professor at the Department of Chemistry, Physics and Pharmacy, where he studies sustainable enzymes. But, as he adds, "the chemistry of the future needs be more sustainable, and this also applies to click chemistry."
Together with colleagues, Wu has published a scientific article in which the team describes a new type of sustainable click chemistry.
Traditional click chemistry requires a catalyst to get the desired chemical reaction started (in click chemistry, you want to get some molecules to 'click' together), and that catalyst is copper ions.
"Copper ions are effective as a catalyst, but toxic to living organisms. So, we wanted to find an alternative to copper in click chemistry," explains Wu.
Carolyn Bertozzi, who further developed Morten Meldal's discovery, found a way to avoid the toxic copper ions.
She came up with the idea of changing the shape of the click molecules to the octagon shape, which made them more reactive and able to click together without copper ions. This technique led to her being co-recipient of the Nobel Prize 2022.
"In our proposal for a new kind of click chemistry, we are still working with copper—but in a different way. We incorporate the copper ions into proteins and thus create a metalloenzyme that is already present in our organism. Copper in proteins poses no danger to nature or living organisms," explains Wu.
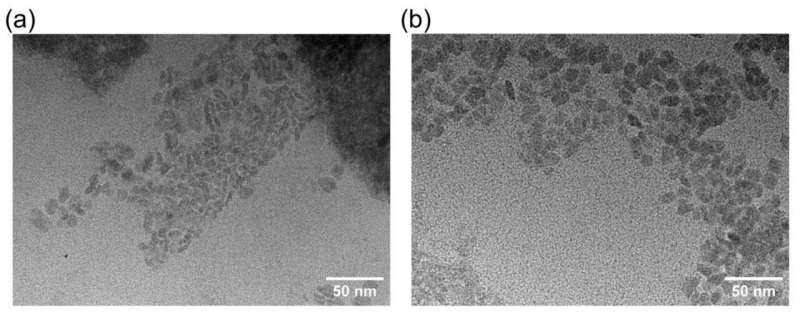
For the task, the research team used cheap and biodegradable proteins, namely bovine serum albumin (BSA), which originates from cows and is a common standard protein in laboratories.
It gets a polymer attached to it, and now copper ions can be embedded in the protein/polymer structure and act as a catalyst to start the click chemistry and allow chemist to put molecules together.
"In short, we use cheap, biodegradable proteins to convert toxic copper ions into non-toxic, biological catalysts," said Wu.
The research is published in Bioconjugate Chemistry.
More information: Ningning Zhang et al, Copper-Containing Artificial Polyenzymes as a Clickase for Bioorthogonal Chemistry, Bioconjugate Chemistry (2022). DOI: 10.1021/acs.bioconjchem.2c00363
Journal information: Bioconjugate Chemistry
Provided by University of Southern Denmark