Niobespherene: A full-metal hollow cage cluster with superatomic stability and resistant to CO attack
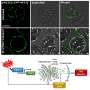
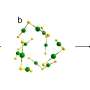
![(Left) Typical mass spectra of the cationic niobium clusters produced by a homemade magnetron sputtering (MagS) source and collected by a customized triple quadrupole mass spectrometer (TQMS), and after reacting with different dose of reactants (3% CO in He). The inset shows the intensity of Nbn(CO)m+ (n=7-16, m=0-8) after Nbn+ clusters react with the CO reactant. (Right) A composition of the superatomic 1S and 1Pz orbitals, with 2S and 2Pz orbitals in Nb12+ contributed by s and d orbitals respectively. Also displayed, are the NICS-scan pertaining to cage aromaticity of the Nb12+ cluster, and the localized orbital locator (LOL) projected at the XY plane. The inserts are the stream traces of the induced ring current of Nb12+ when an external magnetic field is applied in the [0, 0, 1] direction. Credit: Science China Press A full-metal hollow cage cluster Nb12+ niobespherene resistant to CO attack](https://scx1.b-cdn.net/csz/news/800a/2022/a-full-metal-hollow-ca.jpg)
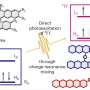
In a paper published in National Science Review, a CO-tolerant niobium cluster Nb12+ was discovered by reacting Nbn+ with CO in a well-designed flow tube reactor.
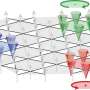
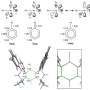
The origin of the chemical inertness of Nb12+, named a "niobespherene," is unveiled by unique superatomic states which are contributed by both the 5s and 4d electrons of niobium. The energy-descent superatomic 2S and 2P orbitals composed of d-electrons delocalize throughout the Nb12+ and balance the cluster structure, giving rise to cage aromaticity and enhanced stability.
This study was led by Dr. Zhixun Luo (State Key Laboratory for Structural Chemistry of Unstable and Stable Species, Institute of Chemistry, Chinese Academy of Sciences).
Transition metal particles are widely applied in a diverse range of fields. However, the precise micro-mechanisms involving universal scaling relationship between intermediates, the formation of metal-metal bonds and collapse of crystal fields are largely unclear by far. A surge of progress in recent years has facilitated new highly detailed studies of metal clusters.
Gas phase metal cluster reactions enable to fully unveil structure-property relationship of nanomaterials and microscopic mechanisms of nano-catalysts at atomic precision. However, it is challenging to prepare pure metal clusters in view of their high activity, thus challenging to experimentally explore their relative stability and property.
Beyond this, it is not always easy to understand why one chemical is more stable than another. A unified answer for metal clusters has led to the establishment of the superatom concept which rationalizes the delocalization of electrons; however, cluster stability based on superatom theory has not been confirmed unambiguously for any metal other than the s- and p-blocks of the periodic table of elements.
Recently researchers in Dr. Luo group have made a great progress in preparing pure metal clusters of niobium. They find a hollow-cage metal cluster Nb12+ which shows up with prominent abundance in the mass spectra after reacting with CO under sufficient gas collision conditions. For the first time, they fully elucidated the superatomic stability of this niobespherene, unveiled the novelty of d-orbital hybridization for forming superatomic orbitals, and illustrated this compound's potential as a CO-tolerant new material.
More information: Benben Huang et al, Nb12+——Niobespherene: a full-metal hollow cage cluster with superatomic stability and resistant to CO attack, National Science Review (2022). DOI: 10.1093/nsr/nwac197
Provided by Science China Press