How do nanoparticles grow? Atomic-scale movie upends 100-year-old theory
For decades, a textbook process known as "Ostwald ripening," named for the Nobel Prize-winning chemist Wilhelm Ostwald, has guided the design of new materials including nanoparticles—tiny materials so small they are invisible to the naked eye.
According to this theory, small particles dissolve and redeposit onto the surface of large particles, and the large particles continue to grow until all of the small particles have dissolved.
But now, new video footage captured by Berkeley Lab scientists reveals that nanoparticle growth is directed not by difference in size, but by defects.
The scientists recently reported their findings in the journal Nature Communications.
"This is a huge milestone. We are rewriting textbook chemistry, and it's very exciting," said senior author Haimei Zheng, a senior scientist in Berkeley Lab's Materials Sciences Division and an adjunct professor of materials science and engineering at UC Berkeley.
For the study, the researchers suspended a solution of cadmium sulfide (CdS) nanoparticles with cadmium chloride (CdCl2) and hydrogen chloride (HCl) in a custom liquid sample holder. The researchers exposed the solution with an electron beam to produce Cd-CdCl2 core-shell nanoparticles (CSNPs)—which look like flat, hexagonal discs—where cadmium atoms form the core, and cadmium chloride forms the shell.
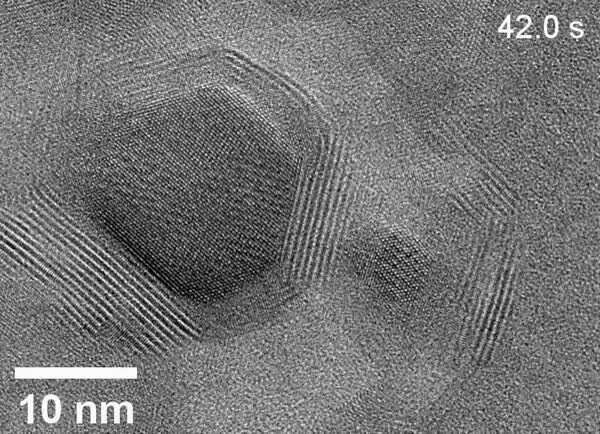
Using a technique called high-resolution liquid cell transmission electron microscopy (LC-TEM) at the Molecular Foundry, the researchers captured real-time, atomic-scale LC-TEM videos of Cd-CdCl2 CSNPs ripening in solution.
In one key experiment, an LC-TEM video shows a small Cd-CdCl2 core-shell nanoparticle merging with a large Cd-CdCl2 CSNP to form a larger Cd-CdCl2 CSNP. However, the direction of growth was guided not by a difference in size but by a crack defect in the shell of the initially larger CSNP. "The finding was very unexpected, but we're very happy with the results," said Qiubo Zhang, first author and postdoctoral researcher in the Materials Sciences Division.
The researchers say that their work is the highest resolution LC-TEM video ever recorded. The advance—monitoring how nanoparticles ripen in solution in real time—was enabled by a custom-made, ultrathin "liquid cell" that secures a tiny amount of liquid between two carbon-film membranes on a copper grid. The researchers observed the liquid sample through ThemIS, a specialized electron microscope at the Molecular Foundry that is capable of recording atomic-scale changes in liquids at a speed of 40-400 frames per second. The microscope's high-vacuum environment keeps the liquid sample intact.
"Our study fills in the gap for nanomaterial transformations that can't be predicted by traditional theory." Zheng said, who pioneered LC-TEM at Berkeley Lab in 2009 and is a leading expert in the field. "I hope our work inspires others to think of new rules to design functional nanomaterials for new applications."
More information: Qiubo Zhang et al, Defect-mediated ripening of core-shell nanostructures, Nature Communications (2022). DOI: 10.1038/s41467-022-29847-8
Journal information: Nature Communications
Provided by Lawrence Berkeley National Laboratory