Cooperative mechanisms of duplicated genes in male differentiation in carp
Polyploidy or whole-genome duplication (WGD) provides extra substrates for genomic evolution and is thus considered as an important driving force for genetic diversity, trait innovation, and ecological adaption. Despite extensive studies on the evolutionary fates and expression patterns of duplicated genes, it remains unclear how duplicated genes co-regulate a biological process in polyploids.
A research group led by Prof. Gui Jianfang from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences recently revealed the cooperative mechanism of two duplicated gonadal somatic cell-derived factor (gsdf) homeologs in male differentiation of hexaploid gibel carp (Carassius gibelio). This study was published in PLoS Genetics.
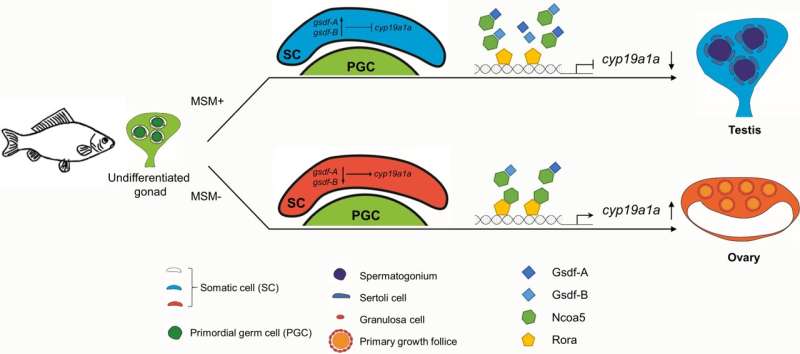
In this study, the researchers identified two gsdf homeologous genes (gsdf-A and gsdf-B) in hexaploid gibel carp, wherein each homeolog contained three highly conserved alleles. They found that Gsdf-A and Gsdf-B were mostly expressed in the somatic cells of the male gonad, and gsdf-A and gsdf-B transcription were mainly activated by dmrt1-A (dsx- and mab-3-related transcription factor 1) and dmrt1-B, respectively.
Then, the researchers performed loss-of-function analysis using CRISPR/Cas9 in the hexaploid C. gibelio with three alleles of gsdf-A and three alleles of gsdf-B. "Loss of either gsdf-A or gsdf-B alone resulted in partial male-to-female sex reversal and loss of both caused complete sex reversal, which could be rescued by an aromatase inhibitor. Compensatory expression of gsdf-A and gsdf-B was observed in gsdf-B and gsdf-A mutants, respectively," said Prof. Gui.
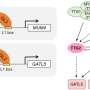
Using yeast two hybrid assay and other molecular methods, the researchers identified that both Gsdf-A and Gsdf-B interacted with Ncoa5 (nuclear receptor coactivator 5) and blocked Ncoa5 interaction with Rora (retinoic acid-related orphan receptor-alpha) to repress Rora/Ncoa5-induced activation of cyp19a1a (cytochrome P450, family 19, subfamily A, polypeptide 1a).
These findings showed that Gsdf-A and Gsdf-B can regulate male differentiation by inhibiting cyp19a1a transcription in hexaploid gibel carp, and can interact with Ncoa5 to suppress cyp19a1a transcription in vitro.
This study provides a typical case of cooperative mechanism of duplicated genes in polyploids and sheds light on the conserved evolution of sex differentiation.
More information: Ming-Tao Wang et al, Two duplicated gsdf homeologs cooperatively regulate male differentiation by inhibiting cyp19a1a transcription in a hexaploid fish, PLOS Genetics (2022). DOI: 10.1371/journal.pgen.1010288
Journal information: PLoS Genetics
Provided by Chinese Academy of Sciences