Bacterial thiosulfate oxidation pathway drives formation of zero-valent sulfur

A research team led by Prof. Sun Chaomin from the Institute of Oceanology of the Chinese Academy of Sciences (IOCAS) verified that Erythrobacter flavus (E. flavus) 21-3 formed zero-valent sulfur (ZVS) in deep-sea cold seep through in situ cultivation.
The study was published in mBio on July 19.
Sulfur is a key element whose transformation and status in the environment are critically dependent upon microbial activities. In their previous study, Prof. Sun's team has found that ZVS is a major sulfur intermediate in the deep-sea cold seep of the South China Sea. They described a novel ZVS-producing pathway determined by thiosulfate dehydrogenase (TsdA) and thiosulfohydrolase (SoxB) mediating the conversion of thiosulfate to ZVS in the deeps-sea E. flavus 21-3.
However, whether in situ bacteria perform sulfur metabolism as they did when cultivated in the laboratory remains unclear.
To investigate whether E. flavus 21-3 produces ZVS in the deep-sea cold seep through the same pathway, the researchers in situ incubated E. flavus 21-3 wild type and mutants missing key genes that determine ZVS formation in the deep-sea cold seep located in the South China Sea for 10 days. They found that E. flavus 21-3 could produce ZVS in the deep-sea cold seep through the same thiosulfate oxidation pathway.
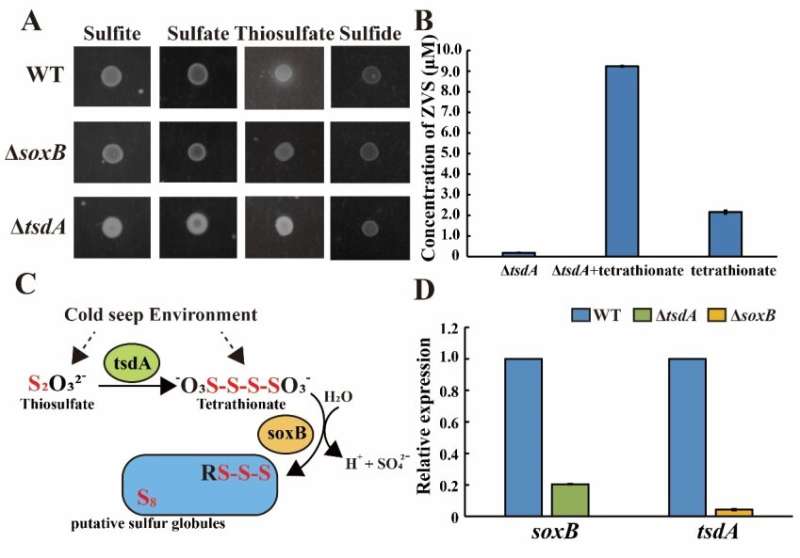
They also compared the distinctions between E. flavus 21-3 cultivated in the deep-sea cold seep and laboratory with/without activating the thiosulfate oxidation pathway. The results showed that this thiosulfate oxidation pathway benefited E. flavus 21-3 to adapt the cold seep conditions. Importantly, ~25% metagenomes assembled genomes derived from the shallow sediments of cold seeps contained both TsdA and SoxB, which presented abundant sulfur metabolism-related genes and active sulfur cycle.
"This suggests that the thiosulfate oxidation pathway determined by TsdA and SoxB exists across many bacteria inhabiting in the cold seep, and it is frequently used by microbes to take part in the active cold seep biogeochemical sulfur cycle," said Prof. Sun.
More information: Ruining Cai et al, Deep-Sea In Situ Insights into the Formation of Zero-Valent Sulfur Driven by a Bacterial Thiosulfate Oxidation Pathway, mBio (2022). DOI: 10.1128/mbio.00143-22
Journal information: mBio
Provided by Chinese Academy of Sciences