Research reveals structure of human endogenous reverse transcriptase
The crystal structure of a human endogenous reverse transcriptase has similarities to HIV reverse transcriptase, a well-known tractable drug target, which will help design drugs to treat cancer and other diseases, according to a study co-authored by a Rutgers researcher.
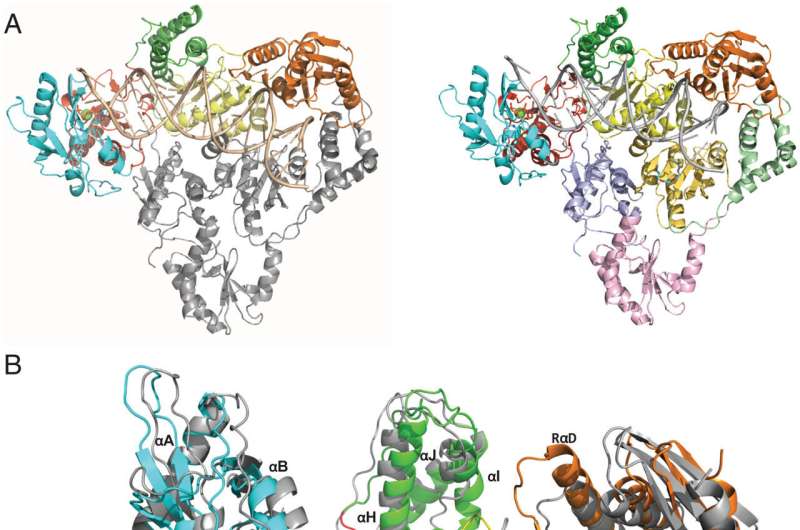
The study, published in the Proceedings of the National Academy of Sciences (PNAS), describes the first-ever high-resolution three-dimensional structure of an endogenous reverse transcriptase—specifically human endogenous retrovirus-K (HERV-K) reverse transcriptase (RT). Past research has found a significant portion of the human genome is made up of repetitive elements that are relics of past viral infections, which are associated with a range of serious diseases, including cancer.
According to the study, the structure provides therapeutic opportunities for RT inhibitors–antiretroviral drugs used to treat HIV infection or AIDS, and also hepatitis B–in cancer, autoimmune and neurodegenerative diseases.
"This study marks a significant step forward in our understanding of endogenous retroviruses and how they could be targeted to treat disease," said Eddy Arnold, resident faculty member at the Rutgers Center for Advanced Biotechnology and Medicine (CABM) and scientific advisory board member of biotechnology company ROME Therapeutics.
"Characterizing the structure of HIV RT was a critical turning point in designing novel medicines to combat that deadly virus," said Arnold, a Distinguished Professor and Board of Governors Professor of chemistry and chemical biology at Rutgers. "Similarly, deeper insights into human endogenous RT could pave the way toward a new class of therapies for cancer and other serious diseases."
Repetitive elements in the genome such as HERV-K are frequently overexpressed in cancer and elicit biological viral mimicry responses that can alter the tumor microenvironment, according to past research.
The study was co-authored by researchers from ROME Therapeutics, a biotechnology company that aims to develop novel therapies for cancer and autoimmune diseases by researching the Dark Genome—vast stretches of uncharted genetic material that represent more than 60 percent of the human genome—for drug development.
"In this publication, we describe for the first time the crystal structure of an endogenous reverse transcriptase, one known as HERV-K RT, and show that it has remarkable similarities to HIV reverse transcriptase, a well-known tractable drug target," said Dennis Zaller, chief scientific officer of ROME. "This achievement is a milestone in the Dark Genome field and sheds light on opportunities for structure-based drug design based on established anti-viral targets present in our human genome. This work is the result of a great collaboration between ROME's exceptional structural biology team and world-leading crystallographers."
More information: Eric T. Baldwin et al, Human endogenous retrovirus-K (HERV-K) reverse transcriptase (RT) structure and biochemistry reveals remarkable similarities to HIV-1 RT and opportunities for HERV-K–specific inhibition, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2200260119
Journal information: Proceedings of the National Academy of Sciences
Provided by Rutgers University