Physical mechanisms explaining DNA and RNA twist changes
The double-helix structure of DNA is deformed by environmental stimuli, which will then affect gene expression, and eventually trigger a sequence of cellular processes. Recent research led by a physicist from City University of Hong Kong (CityU) observed substantial DNA deformations by ions and temperature changes. The researchers developed one simple physical model to explain DNA deformations. These results provide new insights into the molecular mechanisms of cellular responses to ions and temperature changes and can be used to control gene expression using ions and temperature.
Effects of DNA twist changes on gene expression
The research, which was co-led by Dr. Dai Liang, Assistant Professor from Department of Physics, CityU and his collaborators from Wuhan University, focuses on the changes of DNA twist during DNA deformations because the twist is a key structural parameter of the DNA double-helix. Increasing the DNA twist angle (overwinding) not only leads to the formation of DNA supercoils, but also enhances the energy cost of DNA unzipping and hence suppresses gene expression. Note that one key step of gene expression is unzipping a double-stranded DNA into two single-stranded DNA so that the DNA sequence can be read out. On the other hand, decreasing the DNA twist number (unwinding), promotes gene expression. "Active control of DNA twist angle, or DNA supercoils, is employed by bacteria for regulating gene expression," Dr. Dai explained.
Observation of DNA twist changes with salt and temperature
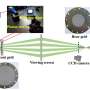
In their research, Dr. Dai and his collaborators observed substantial DNA twist changes when varying the salt concentration and temperature. Their experiments show that DNA twist increases with the rising concentration of sodium chloride (NaCl) and potassium chloride (KCl).
Cracked the mystery of the twist-change mechanism
After observing the intriguing results of salt-induced twist changes, the researchers were motivated to find out the physical mechanisms. Dr. Dai pointed out that the relevant mechanisms are not straightforward due to the various interactions in DNA, such as hydrogen bonds, base stacking, and electrostatic interactions. Varying the salt concentration modifies many interactions in DNA. These interactions affect DNA twist through different pathways and make the final twist change elusive.
Dr. Dai and his collaborators somehow cracked the case. They developed a simple physical model to reveal the mechanism for salt-induced twist change. "We found that more salt strengthens the screening of inter-strand electrostatic repulsion and hence decreases DNA diameter, which eventually increases the twist," Dr. Dai added.
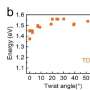
Furthermore, the same physical model quantitatively explains temperature-induced DNA twist changes. Based on the analytical formula of the physical model, the research team derived the variation of DNA twist induced by temperature change, and found that it quantitatively matched the experiment results. It means that two independent phenomena, salt- and temperature-induced DNA twist changes, are driven by the same mechanism.
Their experiment confirmed that a 1°C increase in temperature causes the decrease of DNA twist of 0.01 degrees per base pair. "Don't overlook this '0.01 degree,' such a small twist change per base pair can accumulate along a long DNA, say 1 million base pairs, and causes 10,000 degrees of rotation of about 28 full turns, which would lead to a complicated supercoil," said Dr. Dai.
The findings were published in the academic journal Science Advances.
A unified mechanism for the force-induced twist changes in DNA and RNA
Dr. Dai, Professor Zhang led another related study with Professor Tan Zhijie who is also from Wuhan University. Eventually, they solved a mystery that existed for many years: how does DNA or RNA twist change upon stretching?
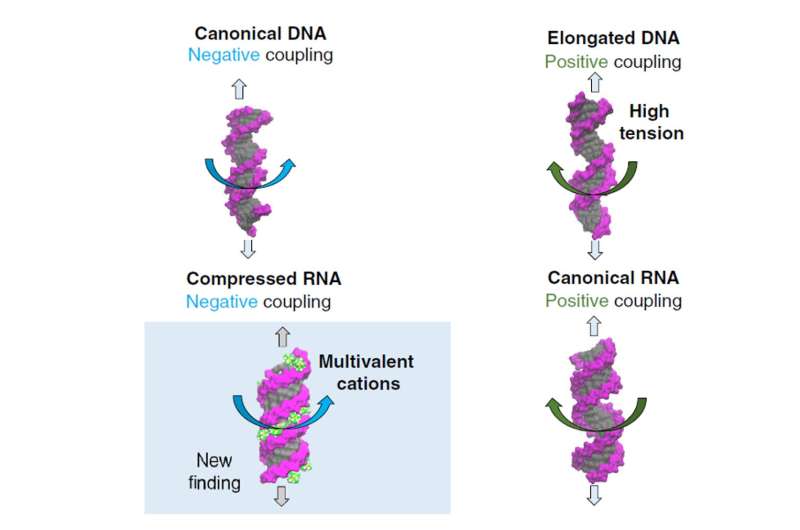
"The answer to it keeps evolving in the past two decades," said Dr. Dai. Scientists expected that stretch should decrease DNA twist. However, an experiment in 2006 observed a counterintuitive trend: stretch increases DNA twist. Later in 2014, another experiment observed that stretch decreases RNA twist, an opposite trend with respect to DNA. "This observation is very surprising, considering that double-stranded RNA and DNA share similar structures but turn out exhibiting opposite responses," said Dr. Dai.
After careful analysis of the force-induced twist changes in DNA and RNA under various conditions, the team found that stretch can both increase and decrease twist in both DNA and RNA, which depends on the situation of DNA or RNA. "Basically, there are four scenarios for DNA and RNA under stretch, while previous studies only observed some of these four," concluded Dr. Dai.
The team came up with a unified mechanism to explain these four scenarios. Stretching canonical DNA and compressed RNA would make them twist more; on the other hand, for elongated DNA and canonical RNA, stretching would make them twist less.
Their findings were published in the academic journal Physical Review Letters.
More information: Chen Zhang et al, Twist-diameter coupling drives DNA twist changes with salt and temperature, Science Advances (2022). DOI: 10.1126/sciadv.abn1384
Xiao-Wei Qiang et al, Multivalent Cations Reverse the Twist-Stretch Coupling of RNA, Physical Review Letters (2022). DOI: 10.1103/PhysRevLett.128.108103
Journal information: Physical Review Letters , Science Advances
Provided by City University of Hong Kong