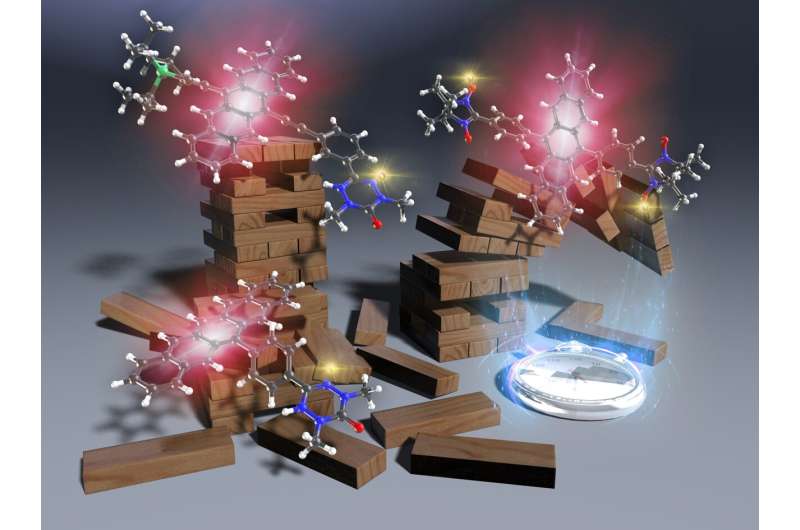
Pentacene derivative has 100 times more light durability than conventional products
Due to high hole mobility, pentacene and its derivatives have been the representative organic semiconductor and have been the subject of much research, both basic and applied. In particular, they are expected to be applied to semiconductor devices, such as field-effect transistors. In addition, organic semiconductors have the advantage of being inexpensive to produce through inkjet printing, and they have low environmental impacts because they do not use metals. However, the backbone of organic semiconductors, such as pentacene, easily reacts with oxygen molecules under visible light, resulting in the loss of useful properties.
A research group led by Professor Yoshio Teki of the Graduate School of Engineering, Osaka Metropolitan University, has achieved photostability more than 100 times higher than that of TIPS-pentacene, a famous commercially-available pentacene derivative, by increasing the planarity of the molecule and strengthening the conjugation of π electrons between a radical substituent and pentacene moiety.
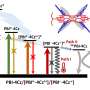
At the same time, to elucidate the mechanism of the remarkable photostability, ultrafast transient absorption measurements using a femtosecond pulsed laser were performed to clarify the peculiar excited-state dynamics of this system. Focusing on the pentacene moiety of the system, they found that intersystem crossing occurs at an ultrafast rate (10-13 seconds), which has never been achieved before in purely organic materials containing no heavy atoms. Furthermore, the subsequent ultrafast deactivation to the ground state was observed to occur within a time of about 10-10 seconds.
"Excellent photostability was achieved by adding a radical substituent that enhances the planarity of the molecules and strengthens the conjugation of π electrons," stated Professor Teki. "In the future, we would like to verify the performance of field-effect transistors and apply them as organic semiconductors."
The study appears in Physical Chemistry Chemical Physics.
More information: Nishiki Minami et al, π-Topology and ultrafast excited-state dynamics of remarkably photochemically stabilized pentacene derivatives with radical substituents, Physical Chemistry Chemical Physics (2022). DOI: 10.1039/D2CP00683A
Journal information: Physical Chemistry Chemical Physics
Provided by Osaka Metropolitan University