Novel zeolites-silver catalyst boosts formaldehyde oxidation at low temperatures
A research group led by Prof. Xiao Jianping and Assoc. Prof. Jiao Feng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. Qu Zhenping from Dalian University of Technology, designed a tandem bifunctional zeolites-silver (Ag) catalyst that could boost formaldehyde oxidation at low temperatures.
This study was published in Nature Communications on April 22.
Theoretical calculations and experimental results showed the activity of formaldehyde oxidation was influenced by the separation between the two components in bifunctional catalyst.
The researchers found a volcano trend for the separation between ZSM-5 and Ag nanoparticles, which means it's not "the closer, the better."
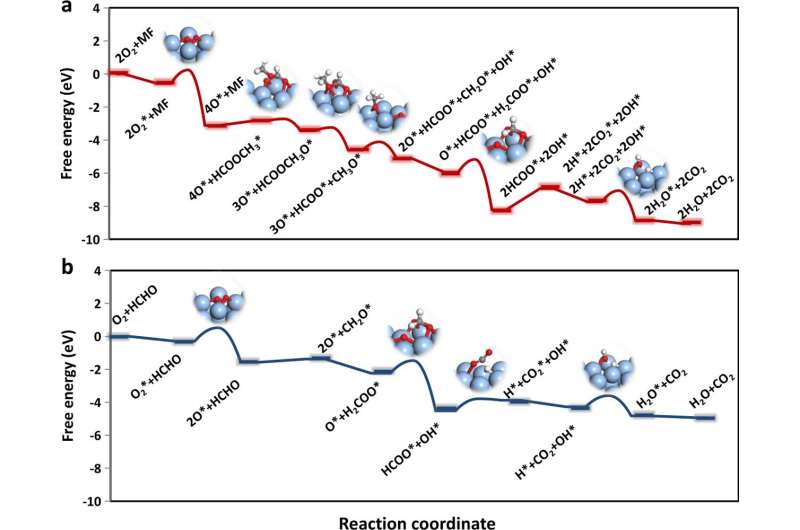
Detached acidic-ZSM-5-activated formaldehyde could generate gaseous intermediates of methyl formate, which was more easily oxidized by subsequent components (Ag). The Ag component would inevitably adsorb unreacted formaldehyde molecules and thereby led to lower methyl formate oxidation activity when the two components were packed too close.
The methyl formate oxidation on Ag components obtained high activity by suppressing the formation of dioxymethylene (DOM), which was hard to be further oxidized.
Compared to that of monofunctional supported silver catalyst, the formaldehyde conversion was increased by 50 times (100% versus 2%) at 70 degrees Celsius.
More information: Na Li et al, Bifunctional zeolites-silver catalyst enabled tandem oxidation of formaldehyde at low temperatures, Nature Communications (2022). DOI: 10.1038/s41467-022-29936-8
Journal information: Nature Communications
Provided by Chinese Academy of Sciences




















