Surfing at the atomic scale: Scientists experimentally confirm new fundamental law for liquids

The first experimental evidence to validate a newly published universal law that provides insights into the complex energy states for liquids has been found using an advanced nuclear technique at ANSTO.
The work has just been published in the Journal of Physical Chemistry Letters as the editor's choice and featured on the front cover of the journal.
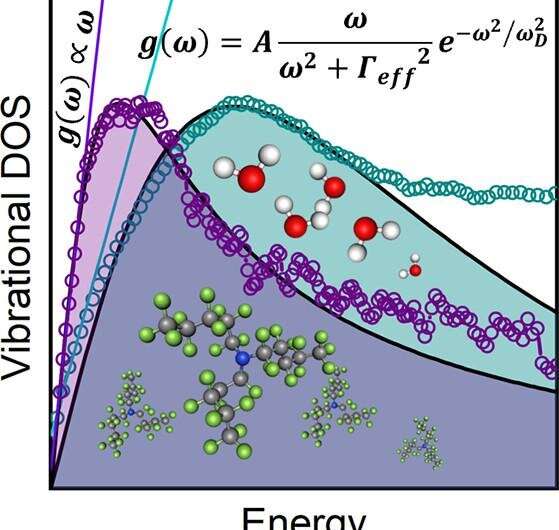
The equation for the vibrational density of states formulated by Alessio Zaccone and Matteo Bagglioli was published in a paper in PNAS in 2021, providing an answer to a question that has been elusive for at least a century.
The elegant mathematical theory has solved the problem of obtaining the distribution of these complex energy states for liquids.
"One of the most important quantities in the physics of matter is the distribution of the frequencies or vibrational energies of the waves that propagate in the material. It is particularly important as it is the starting point for calculating and understanding some fundamental properties of matter, such as specific heat and thermal conductivity, and the light-matter interaction, "said Prof Zaccone on the University of Milan website.
"The big problem with liquids is that, in addition to acoustic waves, there are other types of vibrational excitations related to low energies of the disordered motion of atoms and molecules— excitations that are almost absent in solids. These excitations are typically short-lived and are linked to the dynamic chaos of molecular motions but are nevertheless very numerous and important, especially at low energies. Mathematically, these excitations, known as 'instantaneous normal modes' or INMs in the specialized literature are very difficult to deal with as they correspond to energy states described by imaginary numbers."
The time-of-flight neutron spectrometer Pelican at ANSTO's Center for Neutron Scattering has been used to measure the vibrational densities of states for several liquid systems including water, liquid metal, and polymer liquids. The Pelican instrument has the extreme sensitivity to measure rotational and translational vibrations over short time intervals and at low energies.
The experiments at ANSTO confirmed the linear relationship of the vibrational density of states with frequency at low energies as predicted by Alessio Zaccone and Matteo Bagglioli, as shown in the figure below.
With the COVID lockdown, no accessibility to instruments, the small team that included University of Wollongong Ph.D. candidate Caleb Stamper, Dr. Cortie and Dr. Yu decided to focus on re-analyzing past experimental data from a new perspective, to validate the new law, inspired by the theoretical work from Alessio Zaccone and Matteo Bagglioli.
"The exercise not only achieves such a great outcome but also provides a good introduction of neutron spectroscopy to Caleb, who has done an excellent job," said Dr. Yu as Caleb's ANSTO supervisor and the corresponding author of the paper.
The work would also help them address questions relating to phase transitions in superionic liquids in their work on thermoelectric materials.
"Major challenges arise because liquids are not mechanically stable, as the atoms in a liquid diffuse and the liquid as a whole will flow," explained Dr. Cortie.
The universal law is based on a theoretical framework, known as instantaneous normal modes, as described by Prof Zaccone above, which prescribe a set of instantaneous forces, frequencies, and velocities as quantities.
A complication in deriving a theory to predict the vibrational density of states in liquids arose because of the presence of a small fraction of "imaginary modes."
"Imaginary modes are important because they represent the fact that a liquid is not stable. The atoms in a liquid are strongly interacting with one another all the time but not in the same way a solid does. The relationship is not 'harmonic' meaning that the atoms are not going to be restored to the same configuration after an interaction. The atoms will continue to diffuse quickly and slide past each other," said Stamper.
"The imaginary modes reflect the negative curvature on the potential energy surface of a liquid. It is a very complex energy landscape but if you think of the analogy of a surfer on an ocean wave. The atoms in the liquid follow the curves of the wave itself (see the front cover of the journal). But the atoms can be in a position on the crest, under the surfboard or in the trough, always moving," said Dr. Yu.
"The law will play, for liquids, the same pivotal role that the Debye law plays for solids. It will serve as the foundation for the whole research field involving liquids and beyond."
More information: Caleb Stamper et al, Experimental Confirmation of the Universal Law for the Vibrational Density of States of Liquids, The Journal of Physical Chemistry Letters (2022). DOI: 10.1021/acs.jpclett.2c00297
Alessio Zaccone et al, Universal law for the vibrational density of states of liquids, Proceedings of the National Academy of Sciences (2021). DOI: 10.1073/pnas.2022303118
Journal information: Proceedings of the National Academy of Sciences , Journal of Physical Chemistry Letters