This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Does heat travel differently in tight spaces? New insights into convection heat and fluid mechanics

A search for "air fryer recipe" on most social media platforms likely returns a flood of food videos touting quick and easy meal ideas. The market touts these devices as a convenient, clean, quick way to heat and crispen food, that offers a typically healthier option to using conventional deep fryers.
The technology powering these modern meal machines, however, isn't wholly new. It's based on a simple heating principle found in natural systems and has been used in ovens for decades: convection heat.
Hugo Ulloa, a fluid dynamics scientist at the University of Pennsylvania, notes that convection is driven by temperature gradients creating density differences in a system.
"Picture a pot of water being heated from below; the bottom becomes warmer and less dense, initiating a motion throughout the water body. This process occurs not only in our kitchens but also in diverse environments such as the earth's mantle, oceans, and even our skin," Ulloa says.
"While convection is a well-understood phenomenon in wide-open spaces like the atmosphere or oceans, the behavior of heat in super-confined spaces has remained somewhat of a mystery because it experiences significant changes both in its flow structure and efficiency," he says.
Now, Ulloa, with Daisuke Noto, a postdoctoral researcher in the School of Arts & Sciences, and Juvenal A. Letelier of the University of Chile, has published a paper in the Proceedings of the National Academy of Sciences exploring convection from its smallest scale. The researchers investigated how fluids behave and how heat is transferred in environments that are super-confined, revealing fundamental insights into the rules governing fluid mechanics.
"Daisuke discovered that heat transfer efficiency can be both enhanced or hindered depending on the degree of confinement and the specific flow conditions of the fluid," Ulloa says.
"These findings not only address longstanding issues in our field but could also pave the way for more efficient geothermal energy harvesting, biomedical devices that need precise heat controls to mix compounds or in computer cooling systems, which are becoming increasingly powerful and, as a result, power hungry and dissipating more and more heat."
To explore convection at these new scales, Noto and Ulloa conceptualized and designed a series of experiments using a device known as a Hele-Shaw cell, which consists of two vertically aligned parallel plates with a narrow gap between them, whose interior fluid is heated from the bottom and cooled from above. The gap sizes varied from as small as 2 mm to 4 mm, and the temperature gradients ranged from 1 °C to 30 °C. By manipulating the temperature gradient and the gap size, the scientists were able to observe how heat and fluid motion change as the level of confinement increases.
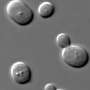
"What we found is fascinating," Noto says. "As we compress the system, we see the emergence of thermal plumes—tiny mushroom-like structures that detach from the boundaries of the base and are fundamental to convection— which can be confined by the lateral walls."
This study builds on the team members' previous work, where they successfully visualized and quantified flow structures in less confined environments. "Our earlier experiments provided the first sound experimental quantification of these flow structures but in more open settings," Ulloa says. "These foundational experiments allowed us to develop the methodologies and theoretical models that we have now applied to these more confined systems."
In explaining the current research, he says the plumes, depending on their size relative to the gap, can either grow freely in a three-dimensional manner or be constrained to a two-dimensional flow.
"This was a long discussion, and Daisuke came with the final brilliant formulation," Ulloa says. "This transition between three-dimensional and two-dimensional flow dramatically affects how heat is transferred. As the gap size decreased, thermal plumes were compressed, resulting in two-dimensional flows that utilize the available energy in transferring heat efficiently.
"However, when the gap was larger than the natural size of the plumes, the plumes grew freely in a three-dimensional manner, resulting in higher but less-efficient heat transfer. This change results from tiny and localized vortical structures created by plumes at the boundaries. What is fascinating is that this small three-dimensional structure living at the boundaries leads to big changes in how heat is transferred. We observed this experimentally and provided a theory for this condition."
This insight allowed the team to develop a new metric, the degree of confinement Λ (lambda), which quantifies the extent of the confinement and its effects on fluid dynamics and heat transfer.
"This research bridges a significant gap in our knowledge," Ulloa says. "We now have a better grasp of how heat transfer behaves in environments that are not fully three-dimensional nor entirely confined like porous media. This understanding is crucial for a range of applications, from geothermal energy extraction to designing more sustainable technologies."
Looking ahead, Ulloa and his team are planning their next study, which builds upon the insights gained from convection at this new scale, as they are focusing on how convective processes in confined systems influence mixing of physical properties like heat and other substances within the fluid, such as minerals, nutrients, or gases like oxygen and methane.
"The next step is to understand not just how heat moves but how other particles and compounds are transported and mixed in these confined environments," Ulloa says.
The new research aims to explore how the mixing of dissolved or suspended substances occurs under varying degrees of confinement and how these processes impact environmental and engineering applications.
"This is particularly important for understanding the distribution of essential nutrients in hydrothermal environments or the efficiency of heat in industrial processes," Ulloa says.
More information: Daisuke Noto et al, Plume-scale confinement on thermal convection, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2403699121
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Pennsylvania