Researchers design a flexible system that sidesteps copper-protein binding
It may seem counterintuitive to many, but metal ions play a critical role in life, carrying out some of the most important biological processes. Think of hemoglobin—a metalloprotein responsible for carrying oxygen to the body's organs via red blood cells. Metalloproteins are proteins bound by at least one metal ion. In the case of hemoglobin, that metal is iron.
For metalloproteins to work properly, they must be paired with the correct metal ion—hemoglobin can only function with iron Yet, protein-metal binding is normally governed by a strict order, called the Irving-Williams Series, which dictates that copper ions should bind to proteins over other metals.
In other words, if a cell contained equal amounts of different metal ions, most cellular proteins and other components would bind to copper, clogging up cellular machinery in the process. This is why organisms spend considerable energy keeping very strict controls over how much free copper is present in cells.
Now researchers in the University of California San Diego's Division of Physical Sciences have reported a new protein-design strategy to sidestep the Irving-Williams Series. The findings were published earlier this week in the journal Nature.
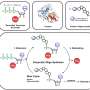
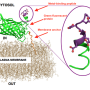
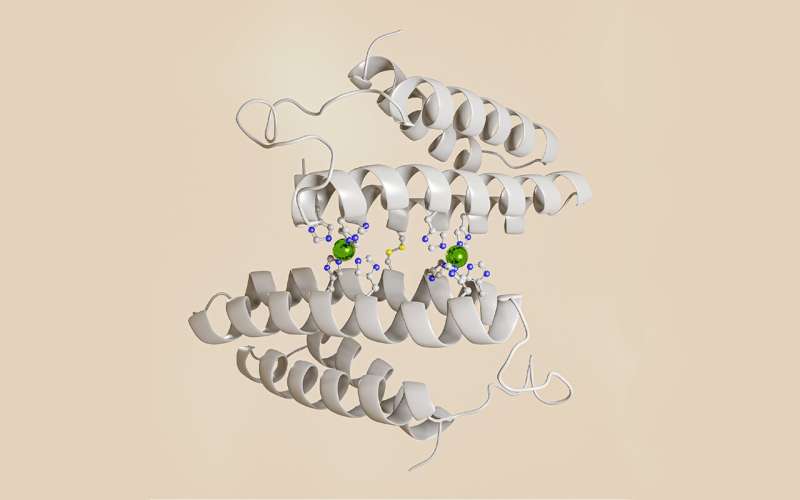
Professor of Chemistry and Biochemistry Akif Tezcan and postdoctoral scholar Tae Su Choi designed a flexible protein that selectively binds other metal ions over copper, paving the way for the design of novel functional proteins and metal sequestration agents. Choi and Tezcan discovered that selective binding to non-copper metals required the artificial protein to present a very specific combination of amino acids and geometries to discriminate against copper. This discovery required an uncommon design approach.
"Protein design typically involves trying to craft a discrete protein structure that can perform a certain function, such as catalysis. This approach is inherently deterministic and follows the sequence of one design-one structure-one function," stated Tezcan. "Best case scenario, you obtain the structure and function that is designed. However, this approach doesn't leave much room for the discovery of new design principles or unexpected outcomes, which are potentially more significant than what was originally planned."
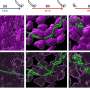
Tezcan and Choi took a probabilistic approach instead. At the outset, their designed protein wasn't engineered to possess a singular structure that selectively binds to a certain type of metal. They created a flexible system that could arrange itself in multiple ways to bind different metal ions in different geometries. It was this flexibility that led them to an outcome they did not originally plan for.
"In analyzing these systems, we saw that proteins were binding to cobalt and nickel ions ahead of copper, which is not the natural order of things," stated Choi. "We created an hypothesis and tested new variants. After extensive analysis, we realized we could construct a protein environment where copper was disfavored."
"This is an example of designing a pathway rather that a target," explained Tezcan. "I personally think that this is a more exciting way to go about the protein design problem. By incorporating an element of flexibility into the design, we leave open the possibility of different outcomes and new design principles we couldn't have known beforehand."
Research on selective metal binding and protein design has importance beyond a better understanding of the fundamentals of life. It can also lay the foundation for more efficient processes during environmental remediation, such as when certain metals need to be sequestered in contaminated water. Protein design is also a critical part of pharmaceutical research and development.
"We were intrigued by the question 'Can we design proteins that can selectively bind to metals or have catalytic reactions in ways that evolution has not yet invented?'" said Choi. "Just because biology doesn't do it, it doesn't mean it's not possible."
More information: Tae Su Choi et al, Overcoming universal restrictions on metal selectivity by protein design, Nature (2022). DOI: 10.1038/s41586-022-04469-8
Journal information: Nature
Provided by University of California - San Diego