March 11, 2022 report
Using electrons to accelerate molecular recognition
A team of researchers affiliated with multiple institutions in the U.S. and China and one in Australia has developed a way to use electrons to accelerate molecular recognition. In their paper published in the journal Nature, the group describes their technique and possible uses for it. Robert Francke, with Leibniz Institute for Catalysis in Germany, has published a News & Views piece in the same journal issue outlining the purpose of molecular recognition research and the work done by the team on this new effort.
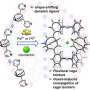
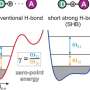
Molecular recognition is a certain type of reaction between molecules where they become connected to one another through non-covalent bonds. Some examples are Van der Waals forces and the stacking of hydrogen bonds. As the researchers note, such processes are difficult to catalyze and because of that there are just a few that are suitable for a few select applications. For that reason, chemists have been searching for a method that could be used more broadly. In this new effort, the researchers have developed a technique that allows for forcing molecular recognition under certain broad conditions—one based on the introduction of electrons.
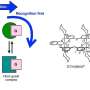
The work by the team involved attempting to induce molecular recognition between two specific molecules—one a ring-shaped macrocycle (to serve as a host), the other a dumbbell shaped molecule (to serve as a guest) with two positive charges (the macrocycle also carried two positive charges). The initial setup was not conducive to molecular recognition. To get them to join, the researchers added a chemical reagent as an electron source in their initial work but later used an electric current. Doing so resulted in the transfer of an electron into the host which reduced its positive charge and decreased the number of unpaired single electrons. And that led to a reduction in repulsion between the guest and the host which allowed them to assemble into an intermediate complex that was not due to a covalent bond. In practice, the dumbbell inserted itself into the macrocycle ring. In such an assemblage, loss of the electron introduced into the host results in decoupling of the two molecules. Francke notes that in addition to giving chemists a new tool for creating bonds between molecules, the new technique could also lead to development of new types of matter.
More information: Yang Jiao et al, Electron-catalysed molecular recognition, Nature (2022). DOI: 10.1038/s41586-021-04377-3
Robert Francke, Self-assembly of molecules triggered by electricity, Nature (2022). DOI: 10.1038/d41586-022-00640-3
Journal information: Nature
© 2022 Science X Network