Controlling ion recognition in reactive host-guest systems
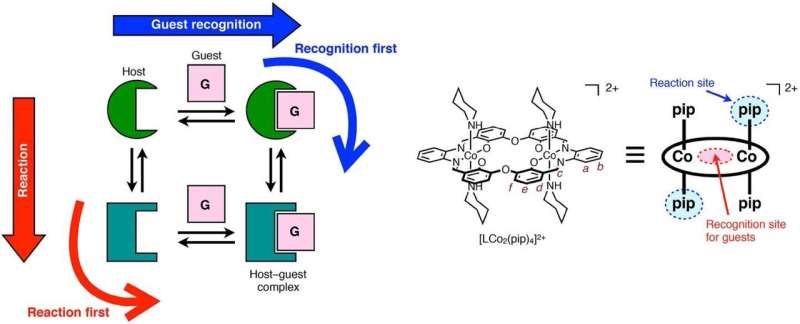
Sometimes a molecule can only undergo a particular chemical reaction if it forms a so-called host-guest complex together with another molecule—the two molecules are then bound together not by covalent bonds but by intermolecular forces. What happens is that first, the host recognizes the guest, after which it can chemically react and become another molecule.
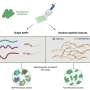
But now, Shigehisa Akine and colleagues from Kanazawa University have shown that the reversed order is also possible: first, the host undergoes a chemical reaction, after which it recognizes and forms a complex with the guest ion. Moreover, they found that the order of recognition and reaction can be switched by modifying the guest ion. Distinguishing between the two alternatives ('recognition first' or 'reaction first') becomes important when the timescale on which the two processes happen differ significantly, a situation that could be exploited in applications including drug delivery.
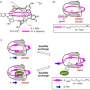
For their study, the researchers used a cobalt-containing host molecule (a 'metallohost'), which has a cavity that can accommodate a particular ion (charged atom) as a guest. The metallohost can undergo reactions of the type known as ligand exchange reaction. The advantage of using this host system is that the occurring reaction processes are slow, and easily monitorable by nuclear magnetic resonance (NMR) spectroscopy. As a guest ion, Akine and colleagues used a compound named NaOTf, containing a sodium ion, which can occupy the host's cavity when forming the host-guest complex.
After adding NaOTf to the metallohost, the NMR signal did not initially point to a structural change. However, after three hours, a change did occur, indicating the formation of new molecules. To determine whether the process was 'recognition first' or 'reaction first', the researchers examined the kinetics of the ligand exchange reaction, and its relation to the sodium concentration. They found that the reaction speed increased significantly with increasing the concentration of sodium, which made them conclude that for sodium the mechanism was 'recognition first'.
Akine and colleagues performed similar experiments with potassium- and rubidium-based guest compounds. Interestingly, they observed that the ligand exchange then occurred in the guest-free form, meaning that the overall process was 'reaction first'.
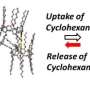
The observed dependence of the type of binding taking place on the type of guest metal ion not only add new insights into host-guest chemistry and their dynamics, but may also lead to applications. The scientists believe that "the understanding of the mechanism would help in developing new time-programmable guest uptake/release systems such as drug delivery systems."
More information: Yoko Sakata et al, Switching of Recognition First and Reaction First Mechanisms in Host–Guest Binding Associated with Chemical Reactions, Journal of the American Chemical Society (2019). DOI: 10.1021/jacs.9b06926
Journal information: Journal of the American Chemical Society
Provided by Kanazawa University