Examining how hydrogen behaves in aluminum alloys
Due to its low density, high strength, and abundance, aluminum and its alloys are widely used for example in constructions, consumer electronics and for vehicles including cars, ships, trains and planes. However, aluminum alloys are prone to hydrogen embrittlement causing catastrophic failure during service if not noticed early enough. Compared to steel, the effects of hydrogen in aluminum are not well understood. Dr. Huan Zhao, postdoctoral researcher at the Max-Planck-Institut für Eisenforschung (MPIE), and her colleagues analyzed how hydrogen embrittles aluminum alloys and found first approaches of hindering this effect. The scientists have now published their latest results in the journal Nature.
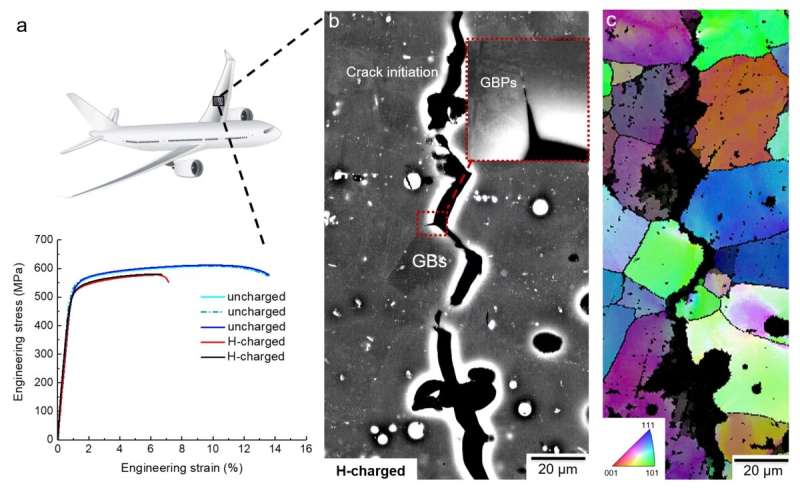
"As hydrogen is the smallest of all elements and has low solubility in aluminum, it is extremely challenging to detect on the atomic scale. Whether and how much hydrogen enters aluminum? Where is it located inside the microstructure and how does it affect the properties? All these were unsolved questions till now," explains Zhao. The MPIE researchers used so-called 7xxx aluminum, a high-strength aluminum class that is the primary material of choice for structural components of airplanes. They charged their samples with hydrogen and performed tensile tests showing that the ductility decreases with increasing amounts of hydrogen.
The fracture surface showed that cracks especially propagated along grain boundaries. Through the cryo-transfer atom probe tomography the scientists revealed that hydrogen gathered along those grain boundaries. "Our experiments were able to show that the amount of hydrogen at particles inside the bulk is much higher than at grain boundaries. However, hydrogen embrittles the material only at the grain boundaries. With the help of computational simulations, we were able to see that hydrogen is attracted by the high energy regions along the grain boundaries causing material's failure and that particles in the bulk rather act as hydrogen traps, which hinder crack propagation," says Dr. Poulami Chakraborty, co-author of the recent publication and postdoctoral researcher at the MPIE.
Intermetallic particles could be a first solution
The MPIE researchers were able to show where hydrogen is located following its ingress during the material's processing or in service. As this cannot really be prevented, it is important to control its trapping. They recommend different strategies to prevent hydrogen embrittlement, in particular through the use of intermetallic particles that could trap hydrogen inside the bulk material. Additionally, control of the magnesium level at grain boundaries appears critical. "Magnesium paired with hydrogen at grain boundaries increases the embrittlement," says Zhao. "At the same time, we must manipulate the correct size and volume fraction of particles in the bulk to trap hydrogen while maintaining the material's strength."
Further studies on the 'perfect' particle distribution and eliminating magnesium decoration of grain boundaries are pursued to design advanced high strength, hydrogen-resistant aluminum alloys.
More information: Huan Zhao et al, Hydrogen trapping and embrittlement in high-strength Al alloys, Nature (2022). DOI: 10.1038/s41586-021-04343-z
Journal information: Nature
Provided by Max Planck Society