February 28, 2022 feature
Experimental evidence for long-distance electrodynamic intermolecular forces
While classical and quantum electrodynamics present the existence of dipole-dipole long-range electrodynamics forces, they remain to be experimentally observed. The discovery of completely new and unanticipated forces that are present between biomolecules have considerable impact to understand the dynamics of molecular machines at work within living organisms. In a new report now published in Science Advances, Mathias Lechelon and a research team at the French National Centre for Scientific Research (CNRS) France conducted two independent experiments based on different physical effects, which they detected via fluorescence correlation spectroscopy and terahertz spectroscopy, respectively, to demonstrate experimental activation of resonant electrodynamic intermolecular forces. The outcomes provided unprecedented experimental proof-of-principle of a physical phenomenon with importance in biology. According to the study, aside from thermal fluctuations that randomly drove molecular motion, resonant and selective electrodynamic forces contributed to molecular encounters in crowded cellular spaces.
Electrodynamic (ED) forces
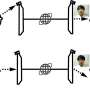
Physicists are interested in observing intermolecular dipole-dipole electrodynamic (ED) forces, and in this study, Lechelon et al. detected these forces at the molecular level. From a physicist's viewpoint, living matter offers a range of fascinating dynamic phenomena, including biomolecules arranged in intricate and complex networks for biochemical interactions to provide astonishing efficiency. Numerically, human cells contain about 130,000 binary interactions between proteins, and about 34,000 unique human protein-protein interactions are listed on databases. One of the most notable challenges in understanding biochemistry from a physics standpoint is to comprehend how the right molecule gets to the accurate place, at the exact moment in time, within the correct cascade of biochemical events during any biological action. Based on existing fundamental interactions, dipole-dipole interactions are predicted to promote molecular attraction due to selective resonance across long distances. In this work, Lechelon et al. experimentally determined the activation of long-range electrodynamic forces between proteins.
Measuring electrodynamic forces in living matter: The experiments
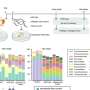
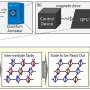
To experimentally determine the activation of long-range electrodynamics forces between proteins, they used R-phycoerythrin; a protein that can be naturally excited via an external energy supply such as a light source. They then worked at different concentrations including intermolecular distances, under the excitation power of a laser light source to observe the activated collective intramolecular oscillation of proteins and their physical mechanisms. During the study, Lechelon et al. used two experimental methods, terahertz spectroscopy with a terahertz rectenna or a microwire probe as sensors and fluorescence correlation spectroscopy. The experimental work supported a proof-of-principle on the capacity for collective oscillations to activate dipole-dipole electrodynamic intermolecular forces to explore the potential of electrodynamic forces in living matter.
Analyzing R-phycoerythrin
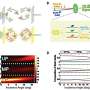
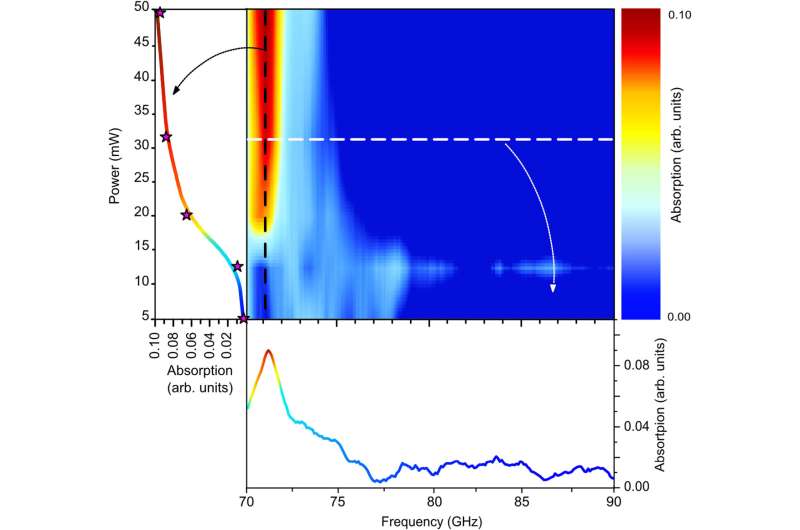
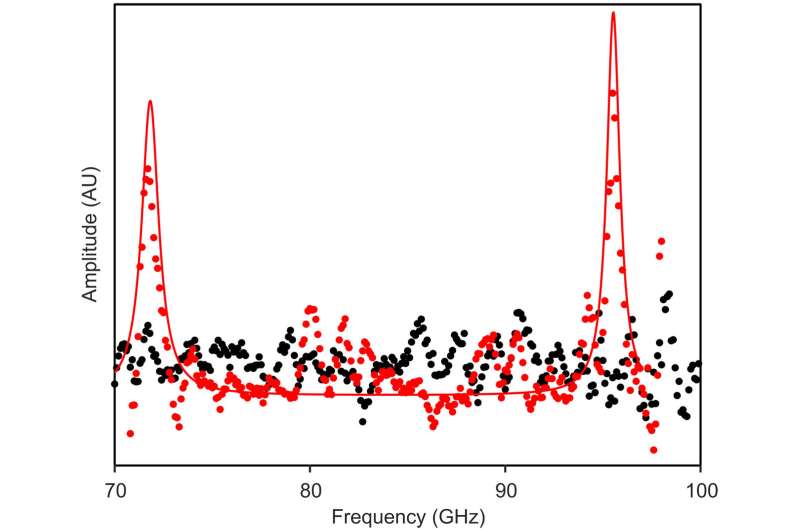
During the initial experiments, Lechelon et al. used a natural light-harvesting protein derived from red algae, known as R-phycoerythrin. The hexamer protein contained an α subunit of two phycoerythrobilins, a β subunit containing three phycoerythrobilins and one phycourobilin and a γ subunit with two or three phycoerythrobilins or one or two phycourobilins. All these subunits contributed to the formation of one of the brightest fluorescent dyes with a sensitivity of 488 nm wavelength of light. Under stable illumination, the molecules entered a coherent vibrational state in saline solution. In the saturation regime, the team noted two collective oscillation frequencies for the protein: one at 0.071 THZ and the other at 0.096 THZ. The team schematized R-phycoerythrin as a torus-shaped object with appropriate parameters computed, including its density and Young's modulus.
Observing phase transitions
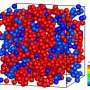
The scientists next obtained a theoretically expected signature of attractive electrodynamic forces among macromolecules vibrating at the same frequency in order to show a phase transition between rapidly diffusing molecules and a clustered phase of very slow moving aggregates of molecules. The team regulated this transition via the average intermolecular distance by adjusting the molecular concentration. During fluorescence correlation spectroscopy experiments, Lechelon et al. varied the protein concentrations and retained the ionic strength of the protein solution at 200 mM via suitable concentrations of salt in water. This ensured good shielding of electrostatic interactions, then by varying the laser power, they repeated typical fluorescence traces for different laser powers and protein concentrations.
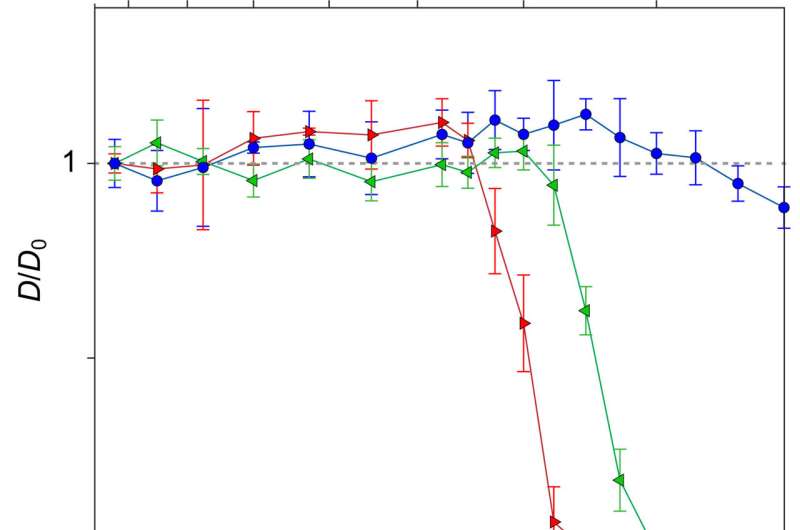
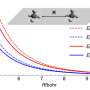
Diffusion coefficients
By measuring the diffusion times from cross-correlation functions of fluorescent traces and by measuring the confocal volume where the molecules are both excited and observed, the team estimated the diffusion coefficients. They then normalized the values of diffusion coefficient relative to Brownian values at a given laser power. Thereafter, the scientists showed how the measured values of diffusion coefficient matched the expected theoretical values at low laser power. For example, at a laser power of 50 µW, the observed diffusion coefficient did not change with the intermolecular distance. For all concentrations considered, the diffusion of R-phycoerythrin molecules were Brownian. Lechelon et al. further conducted molecular dynamics simulations to estimate the effect of long-range electrodynamics interactions for a system of interacting molecules to allow direct comparisons between the outcome of numerical simulations and the outcome of lab experiments. The team noted the appearance of clusters that were theoretically predicted via sudden enhancement of fluorescence fluctuation amplitudes and long transit times using fluorescence spectroscopy.
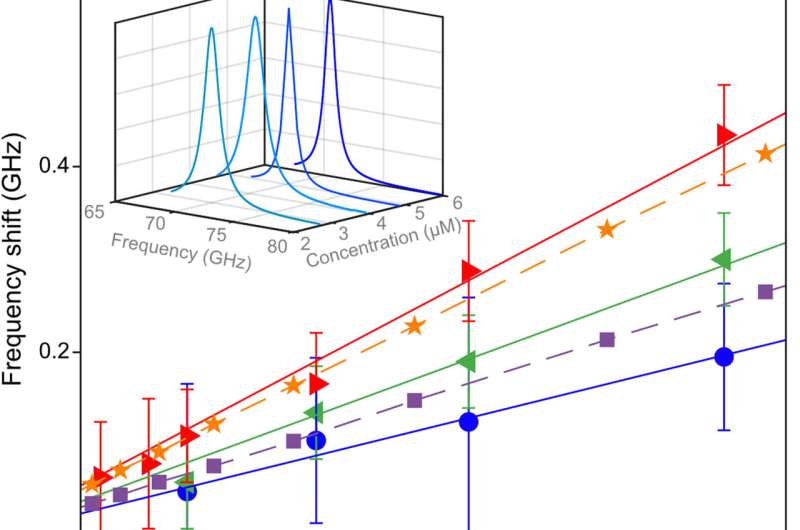
Experimental optimization
By increasing the laser power, Lechelon et al. showed that higher the power, the stronger the oscillation of R-phycoerythrin molecules observed, along with a larger oscillating dipole moment for stronger molecular oscillations. The team could rapidly disaggregate clusters by suddenly lowering the power of the laser light supply in a clustered phase. Under the same conditions of temperature and particle concentration, when the energy input rate was below the threshold, it activated intramolecular collective vibrations, causing the disappearance of clustering transitions, to provide clear proof of the activation of electrodynamic intermolecular attractive forces via collective vibrations of the R-phycoerythrin molecules. Using further studies, Lechelon et al. confirmed the activation of long-distance electrodynamics within bovine serum albumin as well.
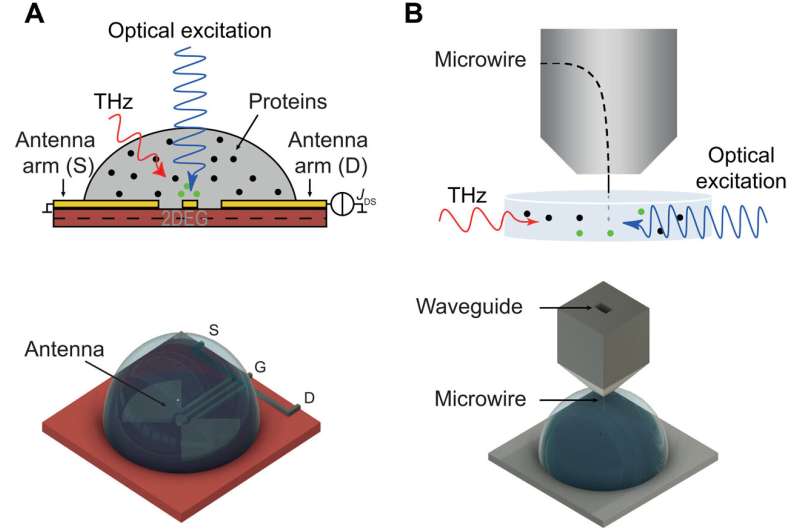
Outlook
In this way, Mathias Lechelon, and colleagues, experimentally showed the excitation of out-of-equilibrium collective oscillations to drive molecular associations via electrodynamic molecular force activation. The R-phycoerythrin proteins incorporated in the work provided convincing evidence for these forces based on complementary experimental approaches. The team consider the protein to be the "Rosetta stone' to identify attractive intermolecular forces.
They activated long-range electrodynamic interactions with a second protein fluorescent dye-labelled bovine serum albumin. The outcomes confirmed the presence of electrodynamic forces in the complex environment encountered in cell biology. Due to their dynamic and reversible nature, electrodynamic forces are instrumental to explain the rationale of many cellular processes. These findings are of interest to regulate the space, time of molecular interactions in living cells and to depict cellular functions. These forces have applications in the domain of drug design to improve therapeutic efficiency and the results may also shed light to overcome adverse effects of molecular overcrowding in cells.
More information: Mathias Lechelon et al, Experimental evidence for long-distance electrodynamic intermolecular forces, Science Advances (2022). DOI: 10.1126/sciadv.abl5855
Kavitha Venkatesan et al, An empirical framework for binary interactome mapping, Nature Methods (2008). DOI: 10.1038/nmeth.1280
Journal information: Science Advances , Nature Methods
© 2022 Science X Network