Research reveals features that influence phase separation
Scientists at St. Jude Children's Research Hospital and Washington University in St. Louis are dissecting the fundamental principles of biological phase separation, a process that is a major mechanism governing how cells are organized. The latest findings highlight the role that protein solubility and charge play in the process. A paper on the work was published today in Nature Chemistry.
Cells sort and separate proteins and other components through phase separation. Interactions among intrinsically disordered proteins, notable for their lack of structure, can drive phase separation. When the process goes awry, it can contribute to neurological diseases and cancer.
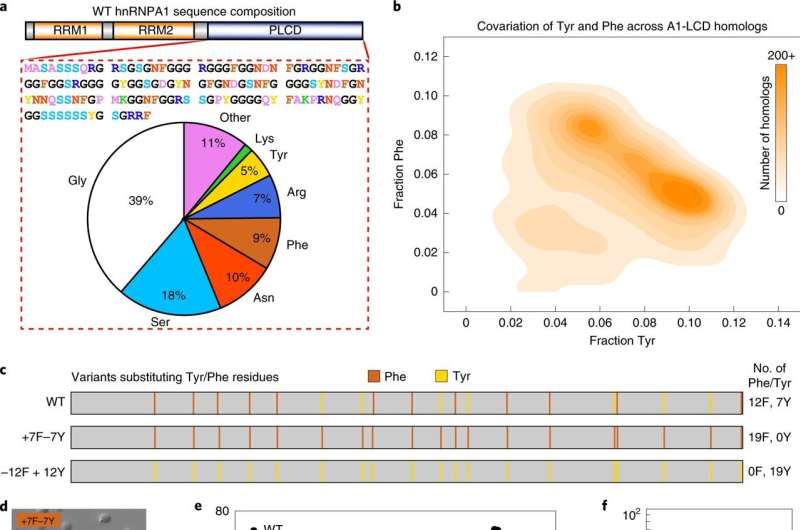
In a previous study, the researchers created a stickers-and-spacers model for phase separation. Stickers are adhesive elements in the DNA sequence, and all other amino acid residues are spacers. The new findings reveal more about what specific stickers and spacers do to drive phase separation, and the role of protein solubility and charge in guiding the process.
"We now have a concept that allows us to know what the stickers and spacers are doing in every sequence context, instead of having to study each individual sequence separately," said co-corresponding author Tanja Mittag, Ph.D., St. Jude Department of Structural Biology. "For the spacers, we wanted to understand what these charged residues specifically do, but also how the physico-chemical features that are determined by the conserved composition of the intrinsically disordered proteins drive phase behavior."
"This work showcases the hidden complexities inherent to low-complexity domains," said co-corresponding author Rohit Pappu, Ph.D., Washington University in St. Louis. "Hierarchies of interaction strengths are encoded at the sequence level and utilized to generate emergent behaviors such as phase separation. With quantitative parsing of the relevant energetics, we can start to use computations to predict the effects of mutations and post-transcriptional processing on the phase behaviors of low-complexity domains, thus moving toward understanding the connections between mutations and disease."
Building on the stickers-and-spacers model
This work builds on prior work by Mittag and Pappu, which led to the stickers-and-spacers model. A paper on that work demonstrated how the interacting regions or stickers in the intrinsically disordered proteins drive phase separation.
The team found that how the stickers are arranged in the sequence and interspersed by spacers is essential for phase separation. Knowing the identity of the stickers and the dimension of the protein chain allowed the team to determine the strength of the sticker-sticker interactions and thus predict protein phase separation.
This new work delves more deeply into the rules that guide phase separation. In particular, they investigated the differences between the specific types of sticker and spacer residues.
"The model is beautifully simple; there are stickers and there are spacers", said co-first author Anne Bremer, Ph.D., St. Jude Department of Structural Biology. "But there is hidden complexity encoded in the sequences. Not all spacers are equal, and they determine phase behavior by how much they like to interact with the solvent."
Natural features that govern phase separation
The researchers identified differences in the behaviors of the stickers tyrosine and phenylalanine, in particular that tyrosine is a stronger sticker. They also found that arginine can be a sticker, in a context-dependent way.
The investigators also showed that the overall positive or negative charge of a protein played a role in how readily it phase separates. The researchers studied a type of intrinsically disordered protein region from RNA-binding proteins. This region tends to contain positively charged arginine residues. The scientists found that some negative charge aids phase separation, but too much reduces it because increased charge per residue increases solubility. This shows that spacers contribute to phase behavior through their effects on solubility.
"When we originally tried to explain phase separation with this model, it wasn't clear why some proteins have stronger or weaker phase behavior," said co-first author Wade Borcherds, Ph.D., St. Jude Department of Structural Biology. "But we found that the higher the overall net charge of a protein, whether it's net positive or net negative, was one of the most important factors in determining how readily it would phase separate."
This work also provides a conceptual basis for understanding modifications that happen after translation, which are important for cell regulation. This includes phosphorylation, which changes the net charge of a protein. Proteins are often aberrantly phosphorylated in disease processes. If multiple phosphorylation events occur across a protein sequence, it can change the driving force for phase separation.
More information: Rohit Pappu, Deciphering how naturally occurring sequence features impact the phase behaviours of disordered prion-like domains, Nature Chemistry (2021). DOI: 10.1038/s41557-021-00840-w. www.nature.com/articles/s41557-021-00840-w
Journal information: Nature Chemistry
Provided by Washington University in St. Louis