Analyzing mushroom DNA to study mutation epistasis

A team of researchers from Skoltech and their Russian and U.S. colleagues have gathered two dozen mushrooms of the species Schizophyllum commune and analyzed their DNA, gaining insights into mutations that cancel the negative effects of other mutations. This phenomenon applies to all organisms but is way more prominent in the extremely genetically variable mushroom than in humans. The mutation interplay enriches natural selection but also points to one of the few disadvantages of sexual reproduction. The preprint is published on biorXiv.
"This study uses the S. commune mushrooms we collected in Michigan, Florida, Moscow, and St. Petersburg to demonstrate a phenomenon called positive epistasis, which involves a harmful mutation being offset by a 'companion' mutation. The resulting survival benefits lead to the mutation pair occurring together more often than it would by sheer chance," the first author of the study, Anastasia Stolyarova of Skoltech, said.
Although epistasis is a fundamental property of genetics that applies to any species, it is very hard to observe in humans, because we are so genetically alike. "Roughly speaking, if you look at the DNA of two randomly selected persons as two strings of letters—A-C-G-T, etc.—they will share something like 999 out of 1,000 letters," Stolyarova explained. "So there is not that much variability, not that much room for mutations that tend to co-occur in the same individual and yet have not become universally present in humans as a species."
Now, Schizophyllum is different. It really takes the notion of variety as the spice of life to a whole new level by allowing for diversity not in every 1,000th but in every fifth letter of the genetic code. "Schizophyllum is what we call the most hyperpolymorphic species there is. It is really bending the notion of what it means to be a Schizophyllum. Even in Drosophila flies, which are a staple of genetic research, it is only every 100th position in the genome that allows for variations," the researcher added.
This means that Schizophyllum must have insane potential for positive epistasis. And it does.
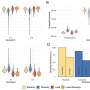
To demonstrate this, the researchers fully sequenced the genome of 54 mushrooms—30 came from a prior study—and analyzed two classes of mutations. Those in the first class are known to be nonfunctional: the idle code in the DNA that we carry around but it does not really affect anything. These mutations were used as controls, because they are fully independent of one another: A useless mutation cannot cancel out or exacerbate another useless mutation. Functional mutations, on the other hand, comprise the other class.
"When we statistically compared the two classes, we could clearly see that functional mutations are inherited pairwise way more often than one would expect at random," Stolyarova said.
Hers and her colleagues' study is the first to identify pairs of mutations engaged in positive epistasis within one species. "This was shown between species, say humans and chimps. Consider a mutation known to cause a disease in humans, yet for chimpanzees, it is the standard variant. Why? Apparently, they'd acquired another mutation long ago that canceled out the bad one going forward. So the two now occur as a pair."
The team also noticed that the co-occurring mutations tended to be localized on the same gene. This makes intuitive sense: Two distinct genes will as a rule be responsible for entirely unrelated proteins, so it is extremely unlikely that changes in the two of them would add up or cancel out through some bizarre interaction.
One insight into evolution that the findings afford is that natural selection can be way more complex than just eliminating the bad traits and retaining the good ones. With the option of one mutation canceling the effects of another, every individual animal takes its own intricate winding path in the grand scheme of evolution.
Curiously enough, another implication of positive epistasis is that we now know for sure that there is one more thing that might go wrong in sexual reproduction. Namely, because it recombines fragments of DNA of the parents, there's always a risk that the offspring might inherit the harmful mutation without its epistatic counterpart and face the negative effects not seen in the parents. That said, this is by no means a game-changing feature for sexual reproduction, which is still the mode of choice for most animals and affords numerous benefits, including but not limited to an edge in adaptability to changes in the environment.
The study demonstrated epistasis by looking at mutations in bulk, statistically analyzing aggregate data, and the researchers did not discuss what the specific functions of those mutations are and how they might cancel each other out. So we may know, for example, that associated mutations reside on a gene responsible for the synthesis of the RadB protein (it plays a part in DNA recombination), but not what their specific functions are and how they interact.
More information: A. V. Stolyarova et al, Complex fitness landscape shapes variation in a hyperpolymorphic species, (2021). DOI: 10.1101/2021.10.10.463656
Provided by Skolkovo Institute of Science and Technology