Double calixarenes bind neuromuscular blockers
Under anesthesia, patients are often given muscle-relaxing neuromuscular blockers to make intubations easier and reduce the skeletal muscle tone during surgery. Using a drug to remove the blocking agent after the operation improves patient recovery and reduces the risk of complications. In the journal Angewandte Chemie, a Canadian research team has now reported a novel broad-spectrum antidote. It consists of two "chalices" that are linked together and cover the two ends of the blocker.
Neuromuscular blockers are drugs that inhibit the transmission of stimuli to the synapses between nerves and muscles by blocking the acetylcholine binding sites on the nicotinic acetylcholine receptors. Different types of blockers meet different pharmacological needs. Antidotes in this class are "drugs that bind other drugs," capturing free blockers in the blood stream and reversing the blockade.
Until now, most "unblockers" have been donut-shaped molecules that encircle the rod-shaped blockers. For this to work the donut hole must be tailored for the thickness of the "rod"—which isn't the same for all types of blocker. Different blockers require different donuts. However, the blockers do share a rodlike structure with two positively charged ends (amino groups), and the rods are all of equal length, because they must simultaneously bridge the gap between two opposite acetylcholine binding cavities.
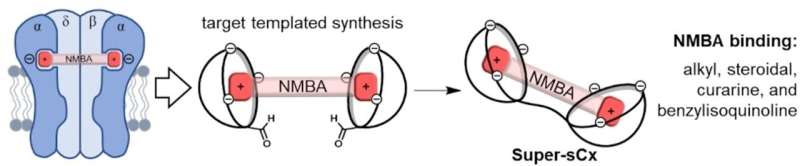
A team at the University of Victoria (Canada) devised a novel approach to make an unblocking agent that can bind a broad spectrum of blockers. Instead of having the rods threaded through a hole, the blocker shields both ends of the rod.
Fraser Hof and his team created cup-shaped molecules known as calix[4]- or calix[5]arenes (calix = chalice). They attached negatively charged groups to the upper rims of the "chalice." Such molecular cups will take up positively charged molecules like the ends of the blocker rod—but unspecifically. To attain selectivity for the blockers, the team wanted to attach two cups to each other by means of a linking segment with a length that exactly matches that of the rod in question—putting the two cups neatly over the two ends.
Because the link needed to be very short, there was repulsion between the two negatively charged chalice rims. The solution was to use a blocker rod as a "template." The team put reactive groups on the chalices and let them bind to a typical blocker. They then used a suitable linker (hydrazine) to tie together the two cups bound to the same blocker rod.
The "double chalices"—Super-sCx4 and Super-sCx5—bind to a broad spectrum of neuromuscular blockers with high selectivity but do not block acetylcholine and other physiologically important amines.
More information: Allison J. Selinger et al, Template‐Directed Synthesis of Bivalent, Broad‐Spectrum Hosts for Neuromuscular Blocking Agents, Angewandte Chemie International Edition (2021). DOI: 10.1002/anie.202113235
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Wiley