Scientists reveal zipper head mechanism of telomere synthesis by human telomerase
A telomere is a region of repetitive nucleotide sequences associated with specialized proteins located at the ends of linear eukaryotic chromosomes to protect the chromosomes from progressive degradation and ensure its integrity. During cell division, telomeres will shorten gradually in human somatic cells, which limits the number of times they can divide. Therefore, telomeres are considered to be closely related to cell aging. Telomerase will be activated during cell division to synthesize telomere DNA for compensating the loss of telomeres.
Recently, a research group led by Prof. Li Guohui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. Lei Ming's and Prof. Wu Jian's group from Shanghai Jiao Tong University School of Medicine, have revealed the key molecular mechanisms of how telomerase repeatedly uses its RNA template to synthesize telomeric DNA. Their study was published in Cell Research on Nov. 15.
Previous studies found that canonical reverse transcriptase (RT) requires a primer-template duplex of at least six base pairs to initiate DNA synthesis, while human telomerase only needs a three-base-paired primer-template duplex to sustain its catalytic activity. The mechanism of the above phenomenon is still unclear.
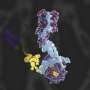
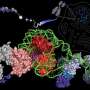
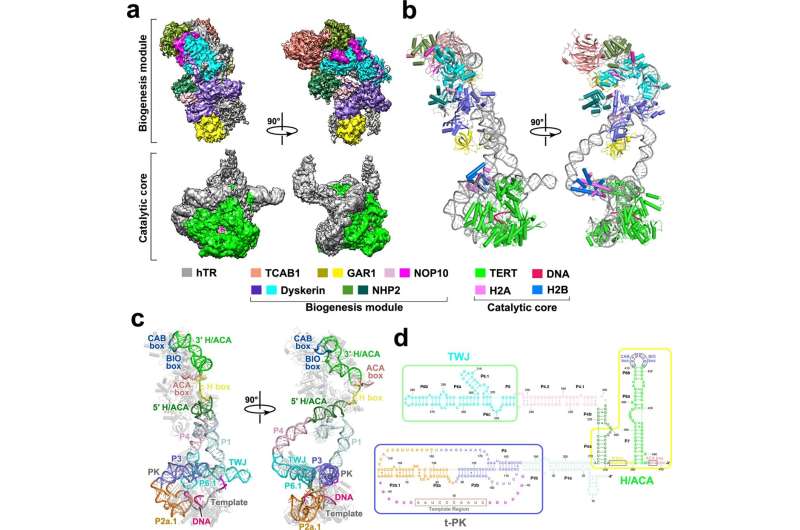
The research group from Shanghai Jiao Tong University School of Medicine identified the cryo-EM structure of human telomerase holoenzyme with bound telomeric DNA at resolutions of 3.54 Å and 3.94 Å for the catalytic core and the biogenesis module, respectively.
Based on this structural information and theoretical studies conducted by Prof Li Guohui's group, this study indicated that the key amino acid Leu980 in the catalytic subunit of human telomerase played a key role in controlling the length and pairing of the DNA-RNA hybrid double helix. The researchers found that Leu980 acted like a zipper head, which separated the substrate and only maintained the three-base-paired primer-template duplex.
Theoretical studies showed that mutation of Leu980 to Gly restored a tighter six-base pair conformation of the DNA-RNA hybrid double. Subsequent biochemical experiments confirmed that the Leu980Gly mutation would affect the continuous and stable DNA synthesis ability of human telomerase.
This work provides novel insights for an in-depth understanding of the telomere repeat synthesis.
More information: Futang Wan et al, Zipper head mechanism of telomere synthesis by human telomerase, Cell Research (2021). DOI: 10.1038/s41422-021-00586-7
Journal information: Cell Research
Provided by Chinese Academy of Sciences