Trapping molecules to find new physics

The Standard Model of particle physics has been extremely successful in describing how the universe works. However, there are some things that it cannot explain. Physicists have, therefore, been looking for new physics in particle accelerators such as the Large Hadron Collider at CERN. At the University of Groningen, a different approach has been used: in contrast to smashing up matter at high energies, physicists wanted to study molecules that are brought to rest. These physicists set a new record by stopping molecules of strontium fluoride, using an electronic trap. Their results were published on 21 October in Physical Review Letters.
According to the Standard Model of particle physics, when the universe started in what is generally known as the "Big Bang," there should have been equal quantities of matter and anti-matter. And these would have canceled each other out. Yet, we live in a universe that is made of matter. "This shows that the fundamental laws of the universe are not as symmetrical as the Standard Model predicts," says Steven Hoekstra, associate professor of Atomic and Molecular Physics at the Van Swinderen Institute for Particle Physics and Gravity of the University of Groningen, in the Netherlands. "And we would like to investigate this asymmetry in a "table top" experiment."
Asymmetry
Other physicists are trying to do the same using particle accelerators such as CERN, in Switzerland. "They smash particles together and study the debris for signs of new physics. We are using a low-energy approach, bringing molecules to a standstill and studying them in detail." The aim of Hoekstra and his research group is to measure the electric dipole moment of an electron. We may think of the electron as just a negative charge, but according to theory, there is a minute asymmetry in its charge when the electron interacts with other particles. "This breaks the symmetry ever so slightly. And we want to investigate this; to see if this symmetry breaking is larger than the Standard Model predicts. In that case, we have proof of physics beyond the Standard Model."
The idea is to study a heavy molecule that is also a free radical that contains an unpaired electron while it is standing perfectly still. This should enable the scientists to use the interactions between this electron and its atomic nucleus as a magnifying glass to determine the electron's electric dipole moment. In theory, this could be done. In practice, it takes a lot of work and determination. Hoekstra has already spent a decade on designing and building a setup for this experiment.

Sensitive
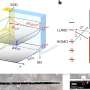
In his laboratory, a 4.5-meter-long construction is sitting on a very long table. At one end, the strontium fluoride molecules are created. "The free radicals that we need are unstable, so producing them is part of the experiment." The molecules are ejected from the first chamber at a speed of 190 meters per second. They then enter a tube in which rings carrying high voltages create a moving electric field. "The rings are tuned to create a spherically symmetric field that moves along with the molecules, trapping them as they go along." The molecules rotate in this trap, like marbles in a bowl, and eventually come to a standstill as the trap is brought to rest. "This happens after four meters. In our latest paper, we show that the molecules are really standing still there."
However, stopping the molecules is one thing. Being able to measure the electric dipole moment of the electrons is something else. "The trap that we created with the electric field changes the energy levels in the molecules. We are studying ways to compensate for this." The first step, therefore, will be to eject the molecules at a speed of 30 meters per second, and use those for a sensitive measurement. "At this speed, the molecules still continue in a straight line, instead of falling down. This allows us to measure the electron's properties for over 10 milliseconds." If these measurements are not sensitive enough to detect the electric dipole moment, they will set new limits to its size.
Stamina
A setup for these measurements is also being developed by Hoekstra and his team. It consists of a large cylinder that shields the molecules from interference by the Earth's magnetic field. "We are currently using this for our first measurements, with a fast beam moving at 600 meters per second. We will then use this with the molecules passing through at only 30 meters per second," explains Hoekstra. He expects that this can happen in about two years from now.
Over the past 10 years, it has also become clear that using barium fluoride, which is slightly heavier than strontium fluoride, will give more accurate results. "So, we'll have to adapt the deceleration to barium fluoride molecules, but this is a relatively small step." The entire project takes a lot of patience and stamina. That is how these ultra-sensitive measurements work. "We have to solve one problem after the other." There are, however, plenty of rewards on the way. The team of some twenty scientists and technicians has now managed to stop strontium fluoride molecules, which are three times heavier than the previous world record, using these techniques. "Now that we can stop them, we need to demonstrate that we can make use of this in our measurements."
More information: P. Aggarwal et al, Deceleration and Trapping of SrF Molecules, Physical Review Letters (2021). DOI: 10.1103/PhysRevLett.127.173201
Journal information: Physical Review Letters
Provided by University of Groningen