Synthesis of new red phosphors with a smart material as a host material

Professor Hiromi Nakano of Toyohashi University of Technology used a material with a unique periodical structure (smart material: Li-M-Ti-O [M = Nb or Ta]) as a host material to synthesize new Mn4+-activated phosphors that exhibit red light emissions at 685 nm when excited at 493 nm. Because the valence of the Mn ions in the material changes from Mn4+ to Mn3+ according to the sintering temperature, composition, and crystal structure, there is a difference in the photoluminescence intensity of the phosphors. XRD, TEM, and XANES were used to clarify the relationship between the photoluminescence intensity and the sintering temperature, composition, crystal structure, and MgO co-doping.
The white color in white LEDs is usually achieved by exciting a yellow phosphor with blue light. However, the color rendering index with this method is assessed as low because there is insufficient red light when compared to sunlight. Therefore, phosphors that emit red light have an important role as materials with a high color rendering index.
Previously, Professor Nakano's team used a smart material (Li-M-Ti-O [M = Nb or Ta]) as a host material to synthesize an Eu3+-activated red phosphor. This time, they synthesized new Mn4+-activated red phosphors without using rare earth materials.
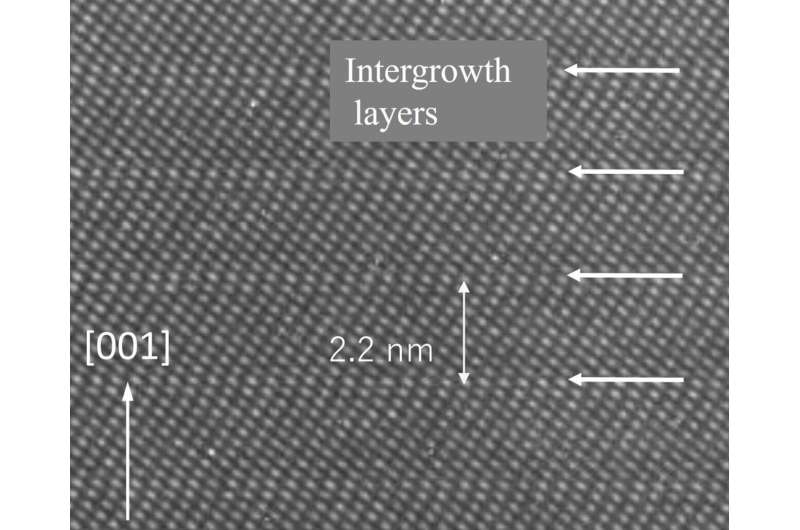
The Li-Nb-Ti-O (LNT) system and Li-Ta-Ti-O (LTT) system are both smart materials (see figure for example) that self-organize into a periodical structure with an intergrowth layer period that changes according to the TiO2 doping amount. The periodical structure area of the LTT system is narrower than that of the LNT system, and there is a difference in the sintering conditions for its creation. Therefore, while comparing the LNT and LTT systems, the team closely investigated how photoluminescence intensity and Mn ion valence change with the sintering temperature, composition, crystal structure, and MgO co-doping.
As a result of this research, it was understood that LTT had notably higher photoluminescence intensity than LNT because of changes in the crystal structure due to the sintering temperature and composition. Generally, if the sintering temperature is high, Mn4+ will likely reduce to Mn3+, explaining the decrease in the photoluminescence intensity. In regards to changes in the crystal structure, when the TiO2 doping amount is increased, the number of [Ti2O3]2+ periodical intergrowth layers also increases. Because the intergrowth layer is formed with Ti3+ ions, it was understood that the surrounding oxygen deficiencies contribute to reductions from Mn4+ to Mn3+. Additionally, when MgO doping was performed to increase the photoluminescence intensity, the LTT phosphor that did not have a periodical structure exhibited a 100% Mn4+ ratio and the highest photoluminescence intensity.
The student who was initially involved in the experiment stated that "the Mn4+ phosphor did not exhibit photoluminescence with the host material", and the research was put on hold for about six months. Next year, a different student synthesized the phosphor and stated, "it exhibits a weak photoluminescence, but I think we could try some things to improve it." Through repeated trial and error, the team uncovered an important factor: in addition to the sintering temperature, there were significant differences in the changes to the crystal structure when the Mn4+ ratio was controlled. Through numerous trips to the Aichi Synchrotron Radiation Center, the team was able to measure the Mn4+ ratio and consolidate their research results.
The Mn4+-activated phosphor had to be synthesized at a comparatively low 850 °C in order to increase the Mn4+ ratio. However, under this condition, there is an issue with moderately low crystallinity. In the future, they will try various co-dopants to further explore the synthesis process to achieve a brighter red phosphor. In recent years, there has been more interest in deep-red Mn phosphors activated without the use of rare-earth materials, such as for use in LED grow lights, and applications can be expected to expand in the future.
More information: Hiromi Nakano et al, Relationship between photoluminescence intensity, Mn ion oxidation, and crystal structure of new phosphors Li-M-Ti-O:Mn4+ (M = Nb or Ta), Materials Research Bulletin (2021). DOI: 10.1016/j.materresbull.2021.111445
Provided by Toyohashi University of Technology