Oxide sintering by air pressure control

Professor Hiromi Nakano of the Toyohashi University of Technology has collaborated with a company to develop a small, lightweight air-pressure control atmosphere furnace that can rapidly and uniformly synthesize periodical structures of Li2O-Nb2O5-TiO2 (LNT) solid solution materials at 3x ordinary pressure. The underlying mechanism was discovered using detailed composition/structure analysis. As the sintering process is reduced by one-fourth compared to conventional electric furnaces, this technology can also be applied to other materials.
The air-pressure control atmosphere furnace is a sintering furnace that uses a regular 100 V AC power outlet and saves up to 800 W of energy. With this furnace, pressurized gas is supplied/controlled using a compressor or gas flow and materials can be heated up to 1,100 degrees C. (FIG. 1)
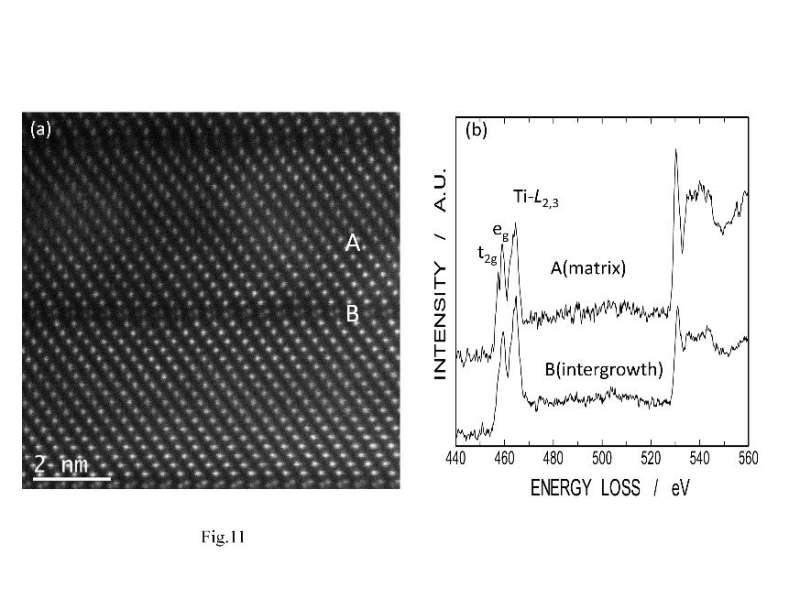
In order to verify the performance of this furnace, the present study focused on LNT solid solutions. Professor Nakano and her team have worked on LNT solid solutions for many years, researching their electrical properties and application as a host material of phosphor, and had already obtained basic data on the material in electric furnaces and millimeter-wave heating systems. Professor Nakano says, "In a particular formation area, this material exhibits a unique periodic structure (superstructure) known as the M-phase in a self-organized formation. This superstructure has a trigonal LiNbO3-type structure as a matrix and is formed by periodically inserting a corundum [Ti2O3]2+ layer as an intergrowth layer so as to divide the matrix." With a conventional electric furnace, materials that have a uniform superstructure require a long sintering process to be synthesized. If these materials could be uniformly synthesized in a shorter period of time, they could be more widely used as practical materials.
How exactly was rapid synthesizing achieved in the present study? It is generally known that an oxygen vacancy mechanism is dominant at low oxygen partial pressures and cation vacancy is dominant at high oxygen partial pressures. Using low gas pressure for this study led the team to discover that there exists an oxygen diffusion mechanism involving interstitial oxygen despite the dominance of cation vacancy. As shown in FIG. 2, Ti valence changes from Ti4+ to Ti3+ at the intergrowth layer to cause oxygen vacancy. Then, interstitial oxygens promote oxygen diffusion along the direction of intergrowth layer just like balls on a pool table. As a result, the grain shapes become anisotropic in the grain growth direction and plate-like grains are formed.
Professor Nakano says, "At the start of development, I considered rapid sintering using a different device because I believed there was no way rapid sintering could be performed using an air-pressure control furnace at approximately 3x the ordinary pressure. But one day, an engineer at our research partner company Full-Tech Co. Ltd., carried out an experiment using this furnace. Even though no similar experiments had been successful in the past, that particular experiment on that particular day produced a very even material. From then on, I started to conduct experiments in this air-pressure control furnace under various conditions to finally confirm a reduction in the sintering process. However, at the time, there were very few reports on successful material synthesis in such pressurized areas, and I spent three months sifting through publications to try and uncover the mechanism behind rapid sintering. It was then that I attended a conference at which one invited speaker talked about oxygen diffusion behavior at high temperatures, showing a video that explained their simulation results. The interstitial oxygen dispersed oxygen ions in a material when the material has oxygen vacancies much like balls on a pool table when struck. As soon as I saw that video, I put two and two together and realized that that was the mechanism behind rapid sintering.
"Currently, we are looking to apply this technology to other materials that take a long time to sinter in an air-pressure control atmosphere furnace. This material can also be used as a material for products in different fields such as optical communication devices, various sensors and LEDs."
More information: Hiromi Nakano et al, Rapid Sintering of Li2O-Nb2O5-TiO2 Solid Solution by Air Pressure Control and Clarification of Its Mechanism, Materials (2018). DOI: 10.3390/ma11060987
Provided by Toyohashi University of Technology