Singlet oxygen selectively degrades oxytetracycline in fenton-like oxidation
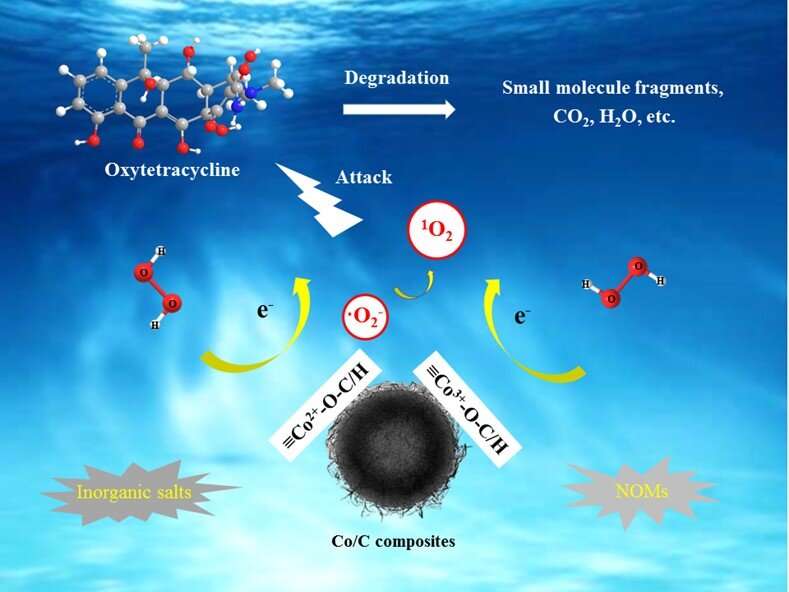
Recently, a research team led by Prof. KONG Lingtao at the Hefei Institutes of Physical Science (HFIPS) of the Chinese Academy of Sciences (CAS) has prepared a type of hollow amorphous Co/C composites to activate hydrogen peroxide (H2O2) to generate singlet oxygen, achieving selective elimination of oxytetracycline (OTC) in complicated water matrices. The relevant results was published in Chemical Engineering Journal.
OTC is the most common tetracycline antibiotic in the field of animal husbandry. It can be detected in water, soil and other areas which features on strong biological stability and cannot be effectively removed by conventional technical means.
As a simple and efficient advanced oxidation technology, Fenton-like oxidation has been considered as an effective way for water pollution. singlet oxygen, as an electrophilic non-radical, exhibits excellent anti-interference to background substrates, and can help to achieve the selective removal of organic pollutants containing electron-rich groups. However, in most Fenton-like reactions, the yield of singlet oxygen is low and the contribution is small.
In this study, the researchers designed and prepared a hollow amorphous Co/C composites with a large number of oxygen-containing functional groups such as carbonyl and hydroxyl distributed on the surface.
They obtained Co/C-3 material by optimizing the ratio of cobalt and carbon, and realized the optimal degradation of 20 ppm OTC by activating H2O2 under neutral pH conditions. The catalytic degradation system exhibited excellent repeatability, stability and anti-interference ability. Quenching experiments and Electron Paramagnetic Resonance results confirmed that converted singlet oxygen was the main oxidizing species, and hydroxyl radical didn't not appear in the system.
The synergistic interaction between cobalt and oxygen-containing functional groups within materials played the key role on activating H2O2 in the formation of singlet oxygen. In addition, the possible degradation pathways and potential ecological toxicities of OTC and its intermediates were revealed.
More information: Peidong Hong et al, Efficient generation of singlet oxygen (1O2) by hollow amorphous Co/C composites for selective degradation of oxytetracycline via Fenton-like process, Chemical Engineering Journal (2021). DOI: 10.1016/j.cej.2021.129594
Provided by Chinese Academy of Sciences