Study reveals new route to rapid, efficient removal of micro-pollutants in water
Recently, the research team led by Prof. Kong Lingtao from Institute of Solid State Physics, Hefei Institutes of Physical Science (HFIPS) prepared a highly active single iron atom catalyst (Fe-ISAs@CN) which can activate HNO2 to generate free radicals, achieving rapid removal of sulfadiazine pollutants in aqueous solutions. The relevant results were published in the Journal of Colloid and Interface Science.
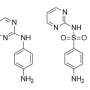
Sulfadiazine (SDZ), a kind of synthetic sulfadiazine antibiotic, is widely used in clinical and animal husbandry industries. However, due to its large-scale use and unqualified discharge of wastewater, more and more antibiotic residues are detected in the water environment. These antibiotics are still highly toxic at very low concentrations. Due to the stable chemical structure of sulfadiazine, it is difficult to solve the residual problem with conventional processing technology.
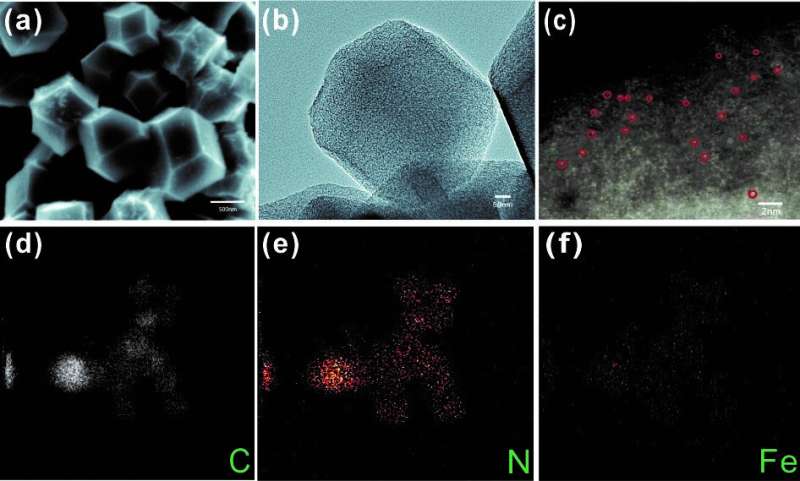
In this research, researchers synthesized the Fe(acac)3@ZIF8 precursor using a solvothermal method, and then calcined at a high temperature of 930 degrees Celsius to prepare a dodecahedral Fe-ISAs@CN catalyst with uniform morphology and good dispersion. Its rough surface and hollow structure provide a large specific surface area and expose a large number of adsorption sites.
The results of degradation experiments showed that 0.1g/L Fe-ISAs@CN could remove 91% of 20 mg/L SDZ within 60 minutes under acid pH conditions.
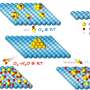
"We looked into the mechanism, and found those active sites could rapidly activate HNO2 in a short time," said Yang Wu, leading scientist of the research, "It produced a large number of active substances with stronger oxidizing energy, and the adsorption site could adsorb SDZ to assist the degradation process."
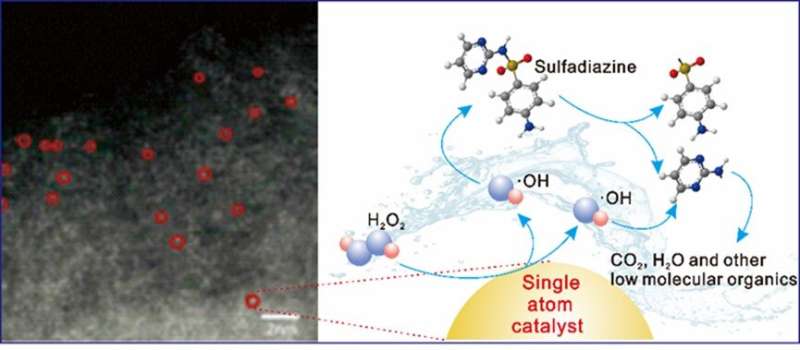
The result proved the rapid degradation of sulfadiazine in the restricted range. Combined with the LC-MS data, they proposed the possible degradation pathways. After five cycles, the removal rate of sulfadiazine was still greater than 80%, and the loss of iron in the catalyst was rather small, indicating good stability of the material.
This work breaks through the traditional Fenton's stringent pH restrictions and provides new ideas for the rapid and deep removal of micro-pollutants in water by nanomaterials.
More information: Wu Yang et al, Enhanced Fenton-like degradation of sulfadiazine by single atom iron materials fixed on nitrogen-doped porous carbon, Journal of Colloid and Interface Science (2021). DOI: 10.1016/j.jcis.2021.03.168
Provided by Chinese Academy of Sciences