Llama 'nanobodies' could hold key to preventing deadly post-transplant infection
Scientists have developed a "nanobody"—a small fragment of a llama antibody—that is capable of chasing out human cytomegalovirus (HCMV) as it hides away from the immune system. This then enables immune cells to seek out and destroy this potentially deadly virus.
Around four out of five people in the UK are thought to be infected with HCMV, and in developing countries this can be as high as 95%. For the majority of people, the virus remains dormant, hidden away inside white blood cells, where it can remain undisturbed and undetected for decades. If the virus reactivates in a healthy individual, it does not usually cause symptoms. However, for people who are immunocompromised—for example, transplant recipients who need to take immunosuppressant drugs to prevent organ rejection—HCMV reactivation can be devastating.
At present, there is no effective vaccine against HCMV, and anti-viral drugs often prove ineffective or have very serious side-effects.
Now, in a study published in Nature Communications, researchers at Vrije Universiteit Amsterdam in the Netherlands and at the University of Cambridge have found a way to chase the virus from its hiding place using a special type of antibody known as a nanobody.
Nanobodies were first identified in camels and exist in all camelids—a family of animals that also includes dromedary, llamas and alpacas. Human antibodies consist of two heavy and two light chains of molecules, which together recognize and bind to markers on the surface of a cell or virus known as antigens. For this special class of camelid antibodies, however, only a single fragment of the antibody—often referred to as single domain antibody or nanobody—is sufficient to properly recognize antigens.
Dr. Timo De Groof from Vrije Universiteit Amsterdam, the study's joint first author, said: "As the name suggests, nanobodies are much smaller than regular antibodies, which make them perfectly suited for particular types of antigens and relatively easy to manufacture and adjust. That's why they're being hailed as having the potential to revolutionize antibody therapies."
The first nanobody has been approved and introduced onto the market by biopharmaceutical company Ablynx, while other nanobodies are already in clinical trials for diseases like rheumatoid arthritis and certain cancers. Now, the team in The Netherlands and the UK have developed nanobodies that target a specific virus protein (US28), one of the few elements detectable on the surface of a HCMV latently infected cell and a main driver of this latent state.
Dr. Ian Groves from the Department of Medicine at the University of Cambridge said: "Our team has shown that nanobodies derived from llamas have the potential to outwit human cytomegalovirus. This could be very important as the virus can cause life threating complications in people whose immune systems are not functioning properly."
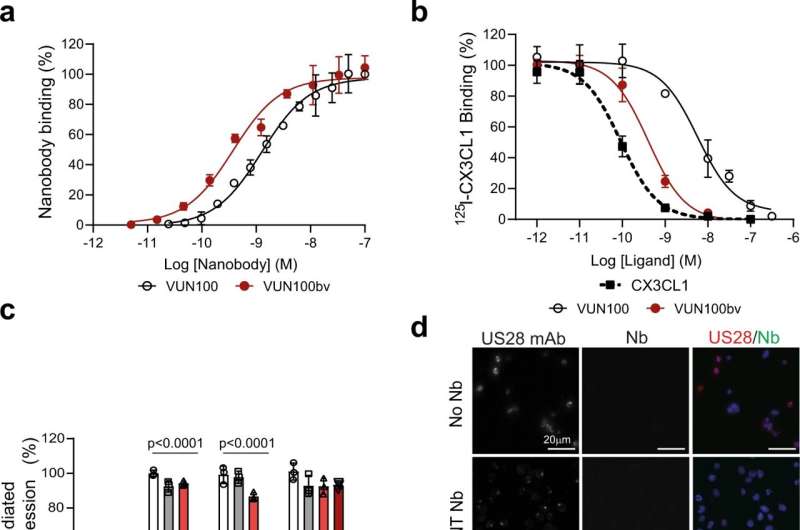
In laboratory experiments using blood infected with the virus, the team showed that the nanobody binds to the US28 protein and interrupts the signals established through the protein that help keep the virus in its dormant state. Once this control is broken, the local immune cells are able to 'see' that the cell is infected, enabling the host's immune cells to hunt down and kill the virus, purging the latent reservoir and clearing the blood of the virus.
Dr. Elizabeth Elder, joint first author, who carried out her work while at the University of Cambridge, said: "The beauty of this approach is that it reactivates the virus just enough to make it visible to the immune system, but not enough for it to do what a virus normally does—replicating and spreading. The virus is forced to put its head above the parapet where it can then be killed by the immune system."
Professor Martine Smit, also from from the Vrije Universiteit Amsterdam, added: "We believe our approach could lead to a much-needed new type of treatment for reducing—and potentially even preventing—CMV infectious in patients eligible for organ and stem cell transplants."
More information: Timo W. M. De Groof et al, Targeting the latent human cytomegalovirus reservoir for T-cell-mediated killing with virus-specific nanobodies, Nature Communications (2021). DOI: 10.1038/s41467-021-24608-5
Journal information: Nature Communications
Provided by University of Cambridge