Researchers first synthesize conjoined bismacrocycle with all phenylene units
The research team led by Prof. Du Pingwu from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) first successfully synthesized an all-phenylene bismacrocycle (bis- means two) with Siamese-twin structure and used fullerene as guest molecules to assemble a peanut-shaped supramolecular complex. This study was published in Angewandte Chemie.
As a new type of carbon material, carbon nanotubes (CNTs) have attracted widespread attention because of their outstanding mechanical and photophysical properties. However, the synthesis of CNTs or CNTs fragments with selective simple structure is still a challenge.
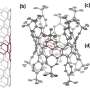
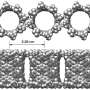
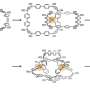
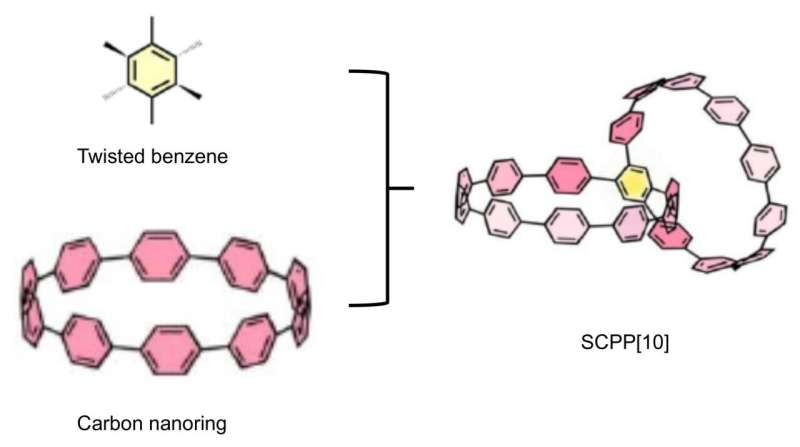
This study reported a conjugated highly strained all-phenylene Siamese-twin bismacrocycle, SCPP[10]. Two phenylene nanorings, [10]CPP, conjoined by a twisted central benzene, showing a unique 3D structure similar to the number 8.
The desired structure is realized through precise select of appropriate building units. Scanning tunneling microscopy (STM) imaging further characterized this conjugated molecule with Siamese-twin shaped structure at the atomic scale.
Researchers applied UV-vis absorption, fluorescence, and theoretical calculations to characterize physical properties of SCPP[10]. Results show that there existed significant redshift of the UV-vis spectrum and fluorescence when compared with the ring component [10]CPP, indicating distinctive nonplanar extended π-conjugated enhancement. Correspondingly, the emission colors of SCPP[10] and [10]CPP were yellow and blue under a hand-held UV lamp.
![Structure (a) and theoretical calculating models (b) of SCPP[10]. STM image (c) shows Siamese-twin shaped structure of SCPP[10]. UV-vis absorption and fluorescence spectra of [10]CPP (the nanoring component of SCPP[10]) and SCPP[10] is shown in (d) and two molecules under UV irradiation at 365 nm. Is shown in (e). Credit: ZHANG Xinyu et al. Researchers first synthesize conjoined bismacrocycle with all phenylene units](https://scx1.b-cdn.net/csz/news/800a/2021/researchers-first-synt-1.jpg)
Besides, the strain energy and largest interphenylene torsion angle of this bismacrocycle were up to 110.59 kcal/mol and 46.07° respectively. Its central benzene had a twisted angle of 10.05°. These novel physical characterizations above were all attributed to the special structure of SCPP[10].
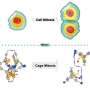
Furthermore, SCPP[10] became a proper supramolecular host due to its curved bismacrocyclic space and suitable diameter. A peanut-shaped 1:2 host-guest complex could be formed after SCPP[10], the peanut shell, capturing two PCBM (fullerene derivatives) molecules as seeds. The binding constants K were also calculated according to the UV-vis titration, and the ratio 1:2 was proved by a Job plot.
![A peanut-shaped 1:2 host-guest complex could be formed after SCPP[10], the peanut shell, capturing two PCBM (fullerene derivatives) molecules as seeds. Credit: ZHANG Xinyu et al. Researchers first synthesize conjoined bismacrocycle with all phenylene units](https://scx1.b-cdn.net/csz/news/800a/2021/researchers-first-synt-2.jpg)
With unique photophysical properties and topology structure, SCPP[10] has been rising as a promising carbon-rich molecule in the photoelectric and supramolecular material field.
More information: Pingwu Du, A Highly Strained All‐Phenylene Siamese‐Twin Bismacrocycle, Angewandte Chemie International Edition (2021). DOI: 10.1002/anie.202104669
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by University of Science and Technology of China